This year, the battery industry celebrates the 25th anniversary of the introduction of the lithium ion rechargeable battery by Sony Corporation. The discovery of the system dates back to earlier work by Asahi Kasei in Japan, which used a combination of lower temperature carbons for the negative electrode to prevent solvent degradation and lithium cobalt dioxide modified somewhat from Goodenough's earlier work. The development by Sony was carried out within a few years by bringing together technology in film coating from their magnetic tape division and electrochemical technology from their battery division. The past 25 years has shown rapid growth in the sales and in the benefits of lithium ion in comparison to all the earlier rechargeable battery systems. Recent work on new materials shows that there is a good likelihood that the lithium ion battery will continue to improve in cost, energy, safety and power capability and will be a formidable competitor for some years to come.

The Electrochemical Society (ECS) was founded in 1902 to advance the theory and practice at the forefront of electrochemical and solid state science and technology, and allied subjects.
ISSN: 1945-7111
JES is the flagship journal of The Electrochemical Society. Published continuously from 1902 to the present, JES remains one of the most highly-cited journals in electrochemistry and solid-state science and technology.
George E. Blomgren 2017 J. Electrochem. Soc. 164 A5019
Toby Bond et al 2025 J. Electrochem. Soc. 172 030512
Electrolyte motion in commercial Li-ion batteries has become an important topic as researchers seek to understand patterns of degradation that occur in large-format cells. Recent work has linked the motion of excess electrolyte to Li plating on the anode of large-format cells after repeated fast charging - an effect known as electrolyte motion induced salt inhomogeneity (EMSI). Mapping the distribution and flow patterns of electrolyte in the cell is critical to understanding these phenomena and predicting the patterns of Li plating that can result. In this work, we used time-resolved, synchrotron computed tomography (CT) to directly image the flow of electrolyte in two commercial 18650 cells during cycling, with one cell containing SiOx in the negative electrode and the other containing only graphite. The former cell shows significantly more electrolyte "pumping" during charge and discharge as well as asymmetric redistribution of salt along the jelly roll after hundreds of cycles. The results yield new insights into how electrolyte motion and its effects are influenced by the composition, geometry, and orientation of the cell.
figure placeholder
Eniko S. Zsoldos et al 2024 J. Electrochem. Soc. 171 080527
Lithium iron phosphate (LFP) battery cells are ubiquitous in electric vehicles and stationary energy storage because they are cheap and have a long lifetime. This work compares LFP/graphite pouch cells undergoing charge-discharge cycles over five state of charge (SOC) windows (0%–25%, 0%–60%, 0%–80%, 0%–100%, and 75%–100%). Cycling LFP cells across a lower average SOC results in less capacity fade than cycling across a higher average SOC, regardless of depth of discharge. The primary capacity fade mechanism is lithium inventory loss due to: lithiated graphite reactivity with electrolyte, which increases incrementally with SOC, and lithium alkoxide species causing iron dissolution and deposition on the negative electrode at high SOC which further accelerates lithium inventory loss. Our results show that even low voltage LFP systems (3.65 V) have a tradeoff between average SOC and lifetime. Operating LFP cells at lower average SOC can extend their lifetime substantially in both EV and grid storage applications.
Manuel Ank et al 2023 J. Electrochem. Soc. 170 120536
Battery research depends upon up-to-date information on the cell characteristics found in current electric vehicles, which is exacerbated by the deployment of novel formats and architectures. This necessitates open access to cell characterization data. Therefore, this study examines the architecture and performance of first-generation Tesla 4680 cells in detail, both by electrical characterization and thermal investigations at cell-level and by disassembling one cell down to the material level including a three-electrode analysis. The cell teardown reveals the complex cell architecture with electrode disks of hexagonal symmetry as well as an electrode winding consisting of a double-sided and homogeneously coated cathode and anode, two separators and no mandrel. A solvent-free anode fabrication and coating process can be derived. Energy-dispersive X-ray spectroscopy as well as differential voltage, incremental capacity and three-electrode analysis confirm a NMC811 cathode and a pure graphite anode without silicon. On cell-level, energy densities of 622.4 Wh/L and 232.5 Wh/kg were determined while characteristic state-of-charge dependencies regarding resistance and impedance behavior are revealed using hybrid pulse power characterization and electrochemical impedance spectroscopy. A comparatively high surface temperature of ∼70 °C is observed when charging at 2C without active cooling. All measurement data of this characterization study are provided as open source.
Peter Keil et al 2016 J. Electrochem. Soc. 163 A1872
In this study, the calendar aging of lithium-ion batteries is investigated at different temperatures for 16 states of charge (SoCs) from 0 to 100%. Three types of 18650 lithium-ion cells, containing different cathode materials, have been examined. Our study demonstrates that calendar aging does not increase steadily with the SoC. Instead, plateau regions, covering SoC intervals of more than 20%–30% of the cell capacity, are observed wherein the capacity fade is similar. Differential voltage analyses confirm that the capacity fade is mainly caused by a shift in the electrode balancing. Furthermore, our study reveals the high impact of the graphite electrode on calendar aging. Lower anode potentials, which aggravate electrolyte reduction and thus promote solid electrolyte interphase growth, have been identified as the main driver of capacity fade during storage. In the high SoC regime where the graphite anode is lithiated more than 50%, the low anode potential accelerates the loss of cyclable lithium, which in turn distorts the electrode balancing. Aging mechanisms induced by high cell potential, such as electrolyte oxidation or transition-metal dissolution, seem to play only a minor role. To maximize battery life, high storage SoCs corresponding to low anode potential should be avoided.
Yuliya Preger et al 2020 J. Electrochem. Soc. 167 120532
Energy storage systems with Li-ion batteries are increasingly deployed to maintain a robust and resilient grid and facilitate the integration of renewable energy resources. However, appropriate selection of cells for different applications is difficult due to limited public data comparing the most commonly used off-the-shelf Li-ion chemistries under the same operating conditions. This article details a multi-year cycling study of commercial LiFePO4 (LFP), LiNixCoyAl1−x−yO2 (NCA), and LiNixMnyCo1−x−yO2 (NMC) cells, varying the discharge rate, depth of discharge (DOD), and environment temperature. The capacity and discharge energy retention, as well as the round-trip efficiency, were compared. Even when operated within manufacturer specifications, the range of cycling conditions had a profound effect on cell degradation, with time to reach 80% capacity varying by thousands of hours and cycle counts among cells of each chemistry. The degradation of cells in this study was compared to that of similar cells in previous studies to identify universal trends and to provide a standard deviation for performance. All cycling files have been made publicly available at batteryarchive.org, a recently developed repository for visualization and comparison of battery data, to facilitate future experimental and modeling efforts.
Chang-Hui Chen et al 2020 J. Electrochem. Soc. 167 080534
Presented here, is an extensive 35 parameter experimental data set of a cylindrical 21700 commercial cell (LGM50), for an electrochemical pseudo-two-dimensional (P2D) model. The experimental methodologies for tear-down and subsequent chemical, physical, electrochemical kinetics and thermodynamic analysis, and their accuracy and validity are discussed. Chemical analysis of the LGM50 cell shows that it is comprised of a NMC 811 positive electrode and bi-component Graphite-SiOx negative electrode. The thermodynamic open circuit voltages (OCV) and lithium stoichiometry in the electrode are obtained using galvanostatic intermittent titration technique (GITT) in half cell and three-electrode full cell configurations. The activation energy and exchange current coefficient through electrochemical impedance spectroscopy (EIS) measurements. Apparent diffusion coefficients are estimated using the Sand equation on the voltage transient during the current pulse; an expansion factor was applied to the bi-component negative electrode data to reflect the average change in effective surface area during lithiation. The 35 parameters are applied within a P2D model to show the fit to experimental validation LGM50 cell discharge and relaxation voltage profiles at room temperature. The accuracy and validity of the processes and the techniques in the determination of these parameters are discussed, including opportunities for further modelling and data analysis improvements.
A. K. Padhi et al 1997 J. Electrochem. Soc. 144 1188
Reversible extraction of lithium from 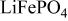 (triphylite) and insertion of lithium into
(triphylite) and insertion of lithium into 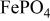 at 3.5 V vs. lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low‐power, rechargeable lithium battery that is inexpensive, nontoxic, and environmentally benign. Electrochemical extraction was limited to ∼0.6 Li/formula unit; but even with this restriction the specific capacity is 100 to 110 mAh/g. Complete extraction of lithium was performed chemically; it gave a new phase,
at 3.5 V vs. lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low‐power, rechargeable lithium battery that is inexpensive, nontoxic, and environmentally benign. Electrochemical extraction was limited to ∼0.6 Li/formula unit; but even with this restriction the specific capacity is 100 to 110 mAh/g. Complete extraction of lithium was performed chemically; it gave a new phase, 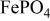 , isostructural with heterosite,
, isostructural with heterosite, 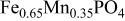 . The
. The 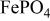 framework of the ordered olivine
framework of the ordered olivine 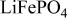 is retained with minor displacive adjustments. Nevertheless the insertion/extraction reaction proceeds via a two‐phase process, and a reversible loss in capacity with increasing current density appears to be associated with a diffusion‐limited transfer of lithium across the two‐phase interface. Electrochemical extraction of lithium from isostructural
is retained with minor displacive adjustments. Nevertheless the insertion/extraction reaction proceeds via a two‐phase process, and a reversible loss in capacity with increasing current density appears to be associated with a diffusion‐limited transfer of lithium across the two‐phase interface. Electrochemical extraction of lithium from isostructural 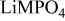 (M = Mn, Co, or Ni) with an
(M = Mn, Co, or Ni) with an 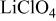 electrolyte was not possible; but successful extraction of lithium from
electrolyte was not possible; but successful extraction of lithium from 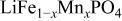 was accomplished with maximum oxidation of the
was accomplished with maximum oxidation of the 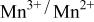 occurring at x = 0.5. The
occurring at x = 0.5. The 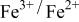 couple was oxidized first at 3.5 V followed by oxidation of the
couple was oxidized first at 3.5 V followed by oxidation of the 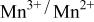 couple at 4.1 V vs. lithium. The
couple at 4.1 V vs. lithium. The 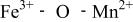 interactions appear to destabilize the
interactions appear to destabilize the 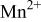 level and stabilize the
level and stabilize the 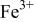 level so as to make the
level so as to make the 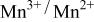 energy accessible.
energy accessible.
Anar Badalbayli et al 2025 J. Electrochem. Soc. 172 032508
This study explores chloride molten salt electrolysis (CMSE) as a promising route for energy-efficient iron metal (Fe) production. Moderate temperature (500 °C) LiCl-KCl molten salts offer excellent thermodynamic stability, high ionic conductivity and diffusivity, and high solubility for FeCl3, thereby enabling efficient Fe metal extraction at high electrowinning rates. Here, we demonstrate the two essential steps for converting taconite ore into Fe metal. First, Fe2O3 from taconite pellets was selectively leached in HCl yielding a high-purity FeCl3 aqueous solution, while the gangue components settled at the bottom. Then, anhydrous FeCl3 was electrolyzed in a LiCl-KCl eutectic molten salt at 500 °C at high current density (1 A cm−2) and at high Coulombic efficiency (>85%). Analysis of the electrowon Fe deposits revealed dendritic structures with purity of >99 wt%, which could be further improved to nearly 100 wt% through arc re-melting. CMSE offers low specific energy consumption (3.7 kWhr kg−1), competitive with H2-DRI and other electrolytic approaches being pursued globally. Our findings underscore the potential of CMSE as an energy-efficient route for electrosynthesis of Fe metal.
Panyawee Bunyanidhi et al 2025 J. Electrochem. Soc. 172 040506
The dissolution of transition metals (TM) from the cathode and their subsequent deposition on the anode represent significant degradation mechanisms in lithium-ion batteries, particularly as the industry seeks to transition towards more sustainable and cost-efficient materials. In this work, the impacts of Mn, Fe, Ni, and Co depositions on the lithiated graphite anode were investigated using pouch storage experiments to simulate the migration-deposition process and compare it to electrodes from real cells. The morphology, chemical distribution, and oxidation states of deposited TMs were investigated by scanning electron microscopy, X-ray absorption spectroscopy, and scanning transmission X-ray microscopy. X-ray diffraction and half-cell studies for post-storage electrodes determined the lithium loss and impedance growth due to TM deposition. The impact of each TM on the lithiated graphite was found to be significantly different. Deposited Mn and Fe were fully metallic, preferred to accumulate on electrode surface, and caused severe delithiation of the graphite, while Ni and Co deposition were rather harmless. The results obtained from simulated TM-containing graphite electrodes closely corresponded with those extracted from cycled cells. This alignment enhances our understanding of the behavior of dissolved TM and paves the way for solutions aimed at mitigating capacity fade in commercial lithium-ion batteries.
Inbal Offen-Polak et al 2025 J. Electrochem. Soc. 172 046506
Hydroxylamine (NH2OH) is a major intermediate in the nitrogen cycle, and its electro-oxidation in alkaline media is key for using nitrogen-based fuels in alkaline fuel cells. Iron- and nitrogen-codoped carbons (FeNCs) are promising electrocatalysts, with Fe-Nx active sites resembling heme (FeN4) centers in enzymes. We report an FeNC catalyst for the hydroxylamine oxidation reaction at E = 0.60 V vs RHE at pH 14. Using a range of control materials, we reveal that the iron plays no significant role in the reaction, whereas the carbon support (reduced graphene oxide) becomes catalytic after a cathodic activation scan. The two voltametric waves for hydroxylamine oxidation on carbon are diffusion-controlled, irreversible, independent of each other, and are more active than either edge or basal plane graphitic sites. Differential electrochemical mass spectroscopy and ion chromatography correlate the oxidation waves with the formation of N2O and NO2 (starting at early potentials) and nitrite and N2 at higher potentials. The electrochemical reactions are coupled with chemical oxidation or disproportionation steps. The reaction mechanism proposed in this study for hydroxylamine oxidation on carbon provides valuable insights for the development of electrocatalysts for nitrogen-based fuels in alkaline devices.
Maria-Lavinia Popa-Cobianu et al 2025 J. Electrochem. Soc. 172 047519
Hepatitis C virus is a widespread persistent viral pathogen that has a global distribution, with a significant infection burden affecting more than 170 million individuals. Determination of the hepatitis C antigen at very low concentrations is essential for its early diagnosis, but also for the assessment of the quality of the transfusion blood. Therefore, two disposable textile sensors based on combined diamond like carbon—Ag nanolayer which was modified with α−, and ß- cyclodextrins were constructed, characterized, and validated for the assay of the hepatitis C virus antigen. The wide working concentration ranges allow the reliable assay of the antigen from 10 ag ml−1 to 1 μg ml−1 and with high sensitivities (6.54 × 1010 and 4.60 × 109 s−1g−1ml when the sensors based on α−, and ß-cyclodextrins, respectively were used for the assay of HCV antigen).The proposed sensors are stable in time and their design is highly reliable. Recoveries higher than 98.00% with relative standard deviation lower than 1.00% were recorded for the HCV from whole blood samples. Student—t test made at 99.00% confidence level, and F test performed at 95.00% confidence level proved the high reliability and accuracy of the method proposed for the assay of HCV antigen.
Ramesh Kumar et al 2025 J. Electrochem. Soc. 172 047517
A non-invasive bio-impedance technique provides a quick response to small changes in the electrical impedance of a phantom or object, making it suitable for agriculture-based applications. This method generates high-frequency, low-current signals that vary with impedance changes in the phantom (e.g., papaya) detected through paired electrodes. The electrodes, positioned at either end of the cylindrical phantom, measure electrical impedance based on voltage changes in response to constant current insertion. Reconstruction algorithms designed in MATLAB generate electrical impedance images using initial conductivity and measured potentials. Electrical Impedance Tomography applies forward and inverse solutions to estimate conductivity distribution within an object, leveraging finite element meshes with triangular elements for computational accuracy. The forward problem involves determining current magnitude in a homogeneous conducting medium. This study developed a GUI-based reconstruction algorithm for the agricultural phantom model using MATLAB. Data acquisition integrates Internet of Things technology, connecting sensors to the GUI system and further to remote monitoring systems. This enables real-time parameter monitoring for agricultural phantom applications. The IoT-based approach demonstrates versatility for agriculture and medical applications, offering efficient, remote-access monitoring of critical parameters.
figure placeholder
Highlights
Develop an IoT-enabled data acquisition system for Electrical Impedance Tomography (EIT)
Developed MATLAB enable system for EIT.
Facilitates real-time parameter monitoring for agricultural applications.
Electrodes positioned at either end of a cylindrical agricultural phantom.
Bihui Li et al 2025 J. Electrochem. Soc. 172 040526
Accurately estimating the capacity of lithium-ion batteries (LIBs) is a critical step in ensuring battery safety. At the moment, the data-driven capacity estimation method is a straightforward and successful strategy. Nevertheless, the issue of missing feature information continues to plague the feature selection process. Additionally, the capacity regeneration phenomena and the mean square error (MSE) loss function's limitations restrict estimation accuracy. To address these drawbacks, this study suggests a novel weighted kernel MSE (WKMSE) loss function, namely AM-LSTM-WKMSE, which combines the attention mechanism (AM) and long short-term memory (LSTM) for LIBs capacity estimation. First, the health indicators (HIs) were extracted from the voltage, current, temperature, and charging/discharging time curves. Following the correlation analysis of HIs, the AM is utilized to generate feature weights, which are subsequently entered into the LSTM. WKMSE is employed as a backpropagation loss function in training. Furthermore, experiments are conducted on the NASA dataset and the CALCE dataset. Comparing the method to alternative approaches, the testing results demonstrate that it has a 0.1% improvement in average the root mean square error of the batteries and superior estimation accuracy. Moreover, the proposed model exhibits strong generalization.
Jingda Wu et al 2025 J. Electrochem. Soc. 172 042505
Rechargeable Al ion batteries (RAIBs) fabricated using Al metal as the anode material have become increasingly popular owing to the advantages of high-abundance, low-cost, and high-capacity of Al metal. AlCl3-based electrolytes have been extensively investigated for use in RAIBs. The high reactivity with moisture and strong corrosivity toward battery components of the AlCl3-based electrolytes have driven researchers to develop active-halide-free (AHF) electrolytes. However, the ability of AHF electrolytes to support reversible Al plating and stripping is yet to be established. Recent studies have suggested that adding BiCl3 to the aluminum trifluoromethanesulfonate-1,2-dimethoxyethane (Al(OTf)3-DME) electrolyte enables reversible Al plating and stripping. Therefore, we investigated the feasibility of Al electrodeposition using the Al(OTf)3-DME electrolyte with and without BiCl3. Cyclic voltammetry and potentiostatic electrolysis indicated that Al electrodeposition is not achieved using the Al(OTf)3-DME electrolyte with and without BiCl3. The addition of BiCl3 only results in the reversible plating and stripping of metallic Bi but not Al. Our study reveals the importance of achieving direct evidence of Al electrodeposition to evaluate whether AHF electrolytes are suitable for use in RAIBs.
Nikita Ahlawat et al 2025 J. Electrochem. Soc. 172 047507
Accurate and sensitive environmental pollution detection is essential for addressing the health risks associated with volatile organic compounds (VOCs) emitted from industrial activities, household products, and vehicles. However, achieving high selectivity and stability under diverse environmental conditions poses significant challenges. Consequently, there is a pressing need for innovative materials that can enhance gas-sensing performance. MXene, a class of two-dimensional transition metal carbides and nitrides, have emerged as promising candidates owing to their exceptional electrical, mechanical, and chemical properties. This review explores various synthesis techniques of MXene, including top-down and bottom-up approaches, highlighting the impact of these methods on the structural and functional properties of MXene. It also examines the combination of MXene with metal oxides to create nanocomposites, focusing on their enhanced performance in VOC sensing applications. The synergistic effect of MXene and metal oxides is analyzed, demonstrating improved sensitivity, selectivity, and response times in VOC detection. MXene-metal oxide nanocomposites use the large surface area, conductivity, and chemical reactivity of MXene, as well as the catalytic capabilities of metal oxides, to provide excellent sensing capability. This article discusses the improved performance of nanocomposites, their production progress, and recent advancements, while also reviewing challenges and providing insights for future research.
Shao-bang Pan et al 2025 J. Electrochem. Soc. 172 044507
As a promising low-cost solar energy conversion technology, dye-sensitized solar cells have attracted widespread attention from researchers due to their low cost, simple preparation method, low toxicity, and easy production. Improving the functional conversion efficiency of batteries has always been an important research direction in this field to obtain more commercial application prospects. Compared to a single photosensitive molecule, the combined action of multiple photosensitizers sometimes exhibits superior photovoltaic performance. In order to provide people with a better understanding of the extraordinary changes brought about by co-sensitization, this article introduces the research results of co-sensitization of various photosensitive dyes with N719 in the past 10 years from different perspectives according to the types of anchoring groups (cyanoacrylic acid, carboxyl group, rhodanine acetic acid, pyridine, and cyanoethylene benzoic acid), hoping to assist with further research on co sensitization mechanisms in the future.
Rongcen Zhao et al 2025 J. Electrochem. Soc. 172 034512
Electrolysis serves as an effective technique for metal preparation, with the electrolytic cell being the fundamental apparatus. The design of the electrolytic cell significantly influences the mass transfer process. Therefore, it is crucial to create an appropriate structure for the electrolytic cell to minimize energy consumption during electrolysis. Given the unique characteristics of the metals involved, the configurations of electrolytic cells vary accordingly. This article examines primary metals produced through electrolysis, such as aluminum and alkali metals, and discusses advancements in research and design principles related to electrolytic cell structures. It also compares various types of electrolytic cells and suggests strategies for structural optimization. Additionally, the role of simulation in the design of electrolytic cells is emphasized. Finally, the article addresses the challenges encountered by electrolytic cells in industrial settings and offers recommendations for structural improvements.
figure placeholder
İsmail Mert Vural and Nurgul K. Bakirhan 2025 J. Electrochem. Soc. 172 037508
The opioid crisis has emerged as a critical public health issue, characterized by the widespread misuse, addiction, and adverse societal impacts of opioid substances. Addressing this multifaceted crisis demands innovative approaches, and the field of forensic science has increasingly turned to electrochemical methods as a powerful tool in the battle against opioids. Here we provide an overview of the significant role played by electrochemical techniques in the detection, analysis, and monitoring of opioids. By harnessing the capabilities of electrochemical sensors, nanomaterial-based platforms, and microfluidic devices, forensic scientists have achieved breakthroughs in opioid detection, offering higher sensitivity, specificity, and rapidity than traditional methods. We explore the latest advancements and applications of electrochemical techniques in forensic opioid analysis, highlighting their potential to revolutionize not only the investigative process but also the management of opioid-related crises. With an emphasis on real-time, on-site, and non-invasive detection, we underscore the importance of electrochemical techniques as a vital component in combating the opioid epidemic and contributing to public safety and well-being.
Alfred B. Anderson 2025 J. Electrochem. Soc. 172 036501
For many years since Gurney introduced quantum mechanics to electrochemistry, models and calculations assumed bonding and other properties at the electrochemical interface may be calculated with adequate accuracy at the potential of zero charge (PZC) and that the effect of potential lies solely in controlling the energy of the electron involved in the transfer, which comes from or goes to an external energy level. The energy of the electron is assigned to the Fermi energy, Ef, of the electrode for the particular potential being modeled. This is done in the Butler-Volmer theory as well as in several quantum mechanical modeling procedures that are introduced here. Though the PZC in fact changes as the identity, amount, and structures of molecules chemically bonded to the electrode are varied during calculations using these models, there is no control of the electrode potential in the calculations. The past two decades have seen the development of computer codes that can incorporate controlled incremental surface charging with polarizable electrolyte models that compensate it, resulting in zero net interface charge. Calculations using these codes provide accurate predictions of the potential-dependent energies of reactants and products, reversible potentials, and electron transfer activation energies.
Maria Kelly et al 2025 J. Electrochem. Soc. 172 046503
Voltammetric measurements of electrochemical CO2 reduction reaction (CO2RR) selectivity on rotating ring disk electrodes (RRDE) are a rapid and sensitive method for quantifying an electrocatalyst's selectivity, i.e. faradaic efficiency (FE). This method has been applied to polycrystalline Au electrocatalysts where a Au disk electrode catalyzes both the CO2RR and hydrogen evolution reaction while the concentric Au ring electrode selectively senses CO by oxidizing CO back to CO2. Such measurements enabled fundamental mechanistic studies but suffer from poor inter-laboratory reproducibility. This work identifies causes of variability in RRDE selectivity measurements by comparing protocols with different electrochemical methods, reagent purities, and glassware cleaning procedures. We observed FECO decrease by 56% during 5 min chronoamperometry measurements, a phenomenon that is not readily apparent in voltammetric scans due to their dynamic nature. Electroplating of electrolyte impurities onto the disk and ring surfaces were identified as a major contributor to Au deactivation. Additionally, the oxygen reduction reaction may lead to higher disk currents in inadequately purged electrolytes, causing an apparent underestimation of FECO at low overpotentials. Lastly, we propose operational bounds for CO2RR selectivity measurements on Au using the RRDE method and provide suggestions on steps for improving the accuracy of this technique.
figure placeholder
Anar Badalbayli et al 2025 J. Electrochem. Soc. 172 032508
This study explores chloride molten salt electrolysis (CMSE) as a promising route for energy-efficient iron metal (Fe) production. Moderate temperature (500 °C) LiCl-KCl molten salts offer excellent thermodynamic stability, high ionic conductivity and diffusivity, and high solubility for FeCl3, thereby enabling efficient Fe metal extraction at high electrowinning rates. Here, we demonstrate the two essential steps for converting taconite ore into Fe metal. First, Fe2O3 from taconite pellets was selectively leached in HCl yielding a high-purity FeCl3 aqueous solution, while the gangue components settled at the bottom. Then, anhydrous FeCl3 was electrolyzed in a LiCl-KCl eutectic molten salt at 500 °C at high current density (1 A cm−2) and at high Coulombic efficiency (>85%). Analysis of the electrowon Fe deposits revealed dendritic structures with purity of >99 wt%, which could be further improved to nearly 100 wt% through arc re-melting. CMSE offers low specific energy consumption (3.7 kWhr kg−1), competitive with H2-DRI and other electrolytic approaches being pursued globally. Our findings underscore the potential of CMSE as an energy-efficient route for electrosynthesis of Fe metal.
Guoxin Li et al 2025 J. Electrochem. Soc. 172 026501
Cathode-electrolyte interphase (CEI) is critical for inhibiting the cathode degradation to maintain cell life. However, the evolution of the CEI is still unclear due to its complex and slow dynamic process. Here we used scanning electrochemical microscopy (SECM) for in situ investigation of CEI formation process on LiFePO4 cathode. Feedback images and probe scan curves showed a heterogeneous passivation that was gently generated on the LiFePO4 particles during both charging and discharging. Besides, a LiFePO4 composited electrode was also used to investigate the CEI formation to simulate the condition of real battery system. The composited cathode does not show obvious CEI formation within first two cycles. The SECM results between the pristine LiFePO4 particles and the composited LiFePO4 indicated the dynamic accumulation of CEI, which is influenced by the ability to charge transfer kinetics of cathode materials. This approach provided a feasible consideration for the connections between the dynamic evolution of the CEI and changes in charge transfer capability of cathode during cycling.
figure placeholder
Highlights
In-situ investigation of cathode-electrolyte interphase formation.
The evolution of native active material and composite slurry were compared.
The electrochemical activity change upon cathode cycling are analysed in situ.
The influence of the charge transfer capability upon CEI generation is revealed.
D. Noel Buckley and Johna Leddy 2024 J. Electrochem. Soc. 171 116503
We revisit the classical derivation of the Butler-Volmer equation to include the effect of the electrode metal. If the metal is replaced by one with a different work function, keeping other conditions in the electrode constant, the chemical potential of electrons  and the Galvani potential
and the Galvani potential  change in a complementary manner. Changes in
change in a complementary manner. Changes in  and
and  each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of
each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of  Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both
Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both  and
and  The exchange current density
The exchange current density  appears as an exponential function of
appears as an exponential function of  For the work function
For the work function  of the metal, the approximation
of the metal, the approximation  yields a linear relationship between
yields a linear relationship between  and
and  The linear increase in
The linear increase in  with
with  has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
figure placeholder
Philip Minnmann et al 2024 J. Electrochem. Soc. 171 060514
The kinetics of composite cathodes for solid-state batteries (SSBs) relies heavily on their microstructure. Spatial distribution of the different phases, porosity, interface areas, and tortuosity factors are important descriptors that need accurate quantification for models to predict the electrochemistry and mechanics of SSBs. In this study, high-resolution focused ion beam-scanning electron microscopy tomography was used to investigate the microstructure of cathodes composed of a nickel-rich cathode active material (NCM) and a thiophosphate-based inorganic solid electrolyte (ISE). The influence of the ISE particle size on the microstructure of the cathode was visualized by 3D reconstruction and charge transport simulation. By comparison of experimentally determined and simulated conductivities of composite cathodes with different ISE particle sizes, the electrode charge transport kinetics is evaluated. Porosity is shown to have a major influence on the cell kinetics and the evaluation of the active mass of electrochemically active particles reveals a higher fraction of connected NCM particles in electrode composites utilizing smaller ISE particles. The results highlight the importance of homogeneous and optimized microstructures for high performance SSBs, securing fast ion and electron transport.
Abdel-Aziz et al
Nitrite concentrations >28.3 µM pose significant risks to human health and the environment. A simple, selective, and cost-effective nitrite sensor was fabricated by electrodeposition of palladium nanoparticles onto the surface of a graphite electrode (nano-Pd/GE). Palladium nanoparticles were characterized using a scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectrometer, revealing an average particle diameter of ~75 nm. For fabrication of nano-Pd/GE, electrodeposition of palladium nanoparticles onto a bare graphite electrode was carried out via cyclic voltammetry. Nano-Pd/GE showed a remarkable electrocatalytic activity towards nitrite oxidation with excellent repeatability and stability. Differential pulse voltammetry was employed under optimized conditions of pH and scan rate for determination of nitrite and exhibited a wide linear response range of 10–2430 µM, high sensitivity of 0.42 μA μM⁻¹ cm⁻², and a low detection limit of 2.0 µM. Selectivity of the nano-Pd/GE electrode for nitrite detection was confirmed, as the presence of common interfering species resulted in only minimal changes in current response. Surface modification of the graphite electrode does not require complex procedures, indicating ease of fabrication. The optimized (nano-Pd/GE) was successfully applied for determination on nitrite in industrial wastewater samples without pretreatment steps, showing high precision and strong agreement with standard methods.
Yue et al
Previous findings for LiFePO4(LFP)/Graphite lithium-ion cells showed that the rate of capacity loss during cycle testing at elevated temperatures is highly dependent on the initial amount of vinylene carbonate (VC) in the electrolyte. To understand the potential mechanism behind this, a series of nominally identical cells with different VC content was prepared and tested for various cycle numbers at 55, 70, 85, and 100°C. Cells were stopped at different cycle numbers to measure gas evolution and charge transfer impedance (Rct) and then disassembled to track VC consumption, Fe deposition on the negative electrode, and diethyl-2,5-dioxahexane carboxylate (DEOHC) generation inside the cell. Higher concentrations of VC improves capacity retention, suppresses the generation of DEOHC and the rapid deposition of Fe on the negative electrode but is accompanied by more rapid gas evolution and Rct increase. Once all of the VC is consumed VC containing cells, Fe deposition accelerates and DEOHC appears in the electrolyte. These findings verify previous suggestions that lithium alkoxide accelerates Fe dissolution and the subsequent Fe deposition on the negative electrode of LFP/Graphite cells.
Upadhyay et al
We report for the first time the application of intrinsic chirality of RRRR (+) - (18-Crown-6) - 2, 3, 11, 12- tetra carboxylic acid ((+) TCA) along with graphene nanosheets (GN) in a chiral carbon paste electrode (CCPE) system for the voltammetric discrimination of tryptophan (TRY) isomers. Embedding (+) TCA in GN enhances rigidity and forms tripodal hydrogen bonds with amine groups of L-TRY and D-TRY, providing the sensor high stereoselectivity. Experimental and quantum chemical computational studies confirmed the stronger affinity of (+) TCA for L-TRY over D-TRY using the differential pulse voltammetry (DPV) and the counterpoise method. The stability constants (1:1 complex) of L-TRY and D-TRY with (+) TCA were 1191 M⁻¹ and 588 M⁻¹, respectively. Additionally, the peak potential difference (ΔEp = EL – ED) between enantiomers on CCPE was 129 mV. The lowest energy conformation of (+) TCA-L-TRY has a Gibbs free energy higher than (+) TCA-D-TRY by 7.08 kcal/mol (B3LYP) or 9.10 kcal/mol (M06-2X), indicating a preference for the L enantiomer. The detection limits for L-TRY and D-TRY are 0.023 µM and 0.075 µM, respectively, with a linear range from 1 µM to 120 µM. The sensor quantified TRY in blood serum and urine samples.
Zhang
Li₂O is one of the most promising lithium ion source materials (LSM) for in-situ prelithiation of SiOx anodes in Li-ion batteries due to its high theoretical lithium-ion capacity (1794 mAh g⁻¹). However, its use faces two major challenges: (1) high Li⁺ extraction over-potential and (2) slight solubility in electrolytes along with high reactivity of oxygen and its oxidation intermediates toward carbonate solvents. To overcome these issues, this work proposes silicon nanoparticles (Si) as absorbents for oxygen and its oxidation intermediates through the reaction “2Li₂O + Si + 4e⁻ → SiO₂ + 4Li⁺”, where the produced SiO₂ is neither soluble in the electrolyte nor reactive with electrolyte solvents and active cathode materials. A 3:1 (wt) Li₂O-Si mixture is shown to reduce the Li⁺ extraction potential to below 4.4 V with a capacity of 1076 mAh g⁻¹, a significant improvement over the 4.8 V and 74 mAh g⁻¹ capacity of pure Li₂O. Additionally, incorporating 2 wt% of the Li₂O-Si additive into the cathode improves the long-term cycling stability of SiOx/NCM811 cells with minimal increases in polarization and capacity loss. These results demonstrate that the Li₂O-Si additive is a highly effective LSM for the in-situ prelithiation of SiOx anodes in Li-ion batteries.
Zhang et al
Detecting dopamine (DA) is vital for understanding neurodegenerative disease and the neurotransmitter-related biological process. Recently, electrochemical sensors have been rapidly developed in many fields due to their high sensitivity, great selectivity, and excellent stability. They are useful to detect and monitor dopamine. In this study, we have constructed a novel electrochemical sensor dependent on the mixing of FeMoSe2 and graphene oxide (GO), and it was used for detecting dopamine. MoSe2 presented the stacking of nanosheets, which provided larger surface areas and more active sites. GO was ultrathin-layered nanostructures, which made it disperse on MoSe2. Because of GO hybridization, the electrochemical activity of FeMoSe2 has been enhanced. In addition, scanning electron microscopy, X-ray diffraction, Fourier transform infrared, and X-ray photoelectron spectroscopy techniques were made use of to characterize the surface morphology and structure of all prepared materials. We also used differential pulse voltammetry technique to assess the sensor’s range of detection (0.04-40 μM). Meanwhile, the sensor displayed the limit of detection of 2.22 nM (S/N = 3). Additionally, the constructed electrochemical sensor exhibited excellent repeatability, stability, and selectivity.
Paul Gasper et al 2025 J. Electrochem. Soc. 172 043509
Early battery life prediction models are most useful for R&D if they help us understand the early changes in battery electrochemical response that correspond with long-term degradation and failure. Linear regression models such as Fused lasso and Partial Least Squares can fit coefficients directly to high-dimensional electrochemical data like capacity-voltage and ΔV–state-of-charge, i.e., Q(V) and ΔV(SOC) curves, learning coefficients that can be physically interpreted. We leverage the ISU-ILCC battery aging data set to learn high-dimensional coefficients for early battery life prediction from traditional slow-rate capacity check data, demonstrating learning on Q(V), dQ·dV−1, and ΔV(SOC) curves. A thorough study on the dependence of coefficient values on train/test size and data preprocessing methods is made, demonstrating the reliability of high-dimensional regression approaches unless very small amounts of data are used for model training. For this data set, coefficients from Q(V) and dQ·dV−1 models highlight changes in electrode stoichiometry due to lithium loss, while ΔV(SOC) coefficients highlight changes in positive electrode diffusivity due to particle cracking as well as electrode stoichiometry shifts. By directly interpreting the coefficients of a regression model, we make physical insights into battery degradation mechanisms without requiring the assumptions of traditional battery data analysis methods.
Yunhao Xiao and Rui Qiao 2025 J. Electrochem. Soc. 172 040525
The growing volume of spent lithium iron phosphate (LFP) batteries underscores the need for efficient recycling to mitigate environmental concerns and recover valuable materials. This study presents an upcycling strategy integrating pre-oxidation treatment and Al-V co-doping to produce regenerated LFP (RLFP) from spent LFP (SLFP). The pre-oxidation process effectively removes residual binders, carbon, and electrolytes, while Al-V co-doping shortens the Li+ migration path, reduces charge-transfer resistance, and enhances the electrochemical performance of RLFP. Notably, one of the RLFPs prepared by co-doping achieves an outstanding discharge capacity of 146.7 mAh g−1 at 1 C, and exceptional cycling stability, retaining 95.4% capacity after 200 cycles at 1 C, which amounts to a 21.5% improvement in capacity retention compared to undoped RLFP. This work thus provides a scalable recycling pathway by offering new insights into impurity control in processing SLFP and highlighting the potential of co-doping to enhance SLFP upcycling.
Meng Yue et al 2025 J. Electrochem. Soc.
Previous findings for LiFePO4(LFP)/Graphite lithium-ion cells showed that the rate of capacity loss during cycle testing at elevated temperatures is highly dependent on the initial amount of vinylene carbonate (VC) in the electrolyte. To understand the potential mechanism behind this, a series of nominally identical cells with different VC content was prepared and tested for various cycle numbers at 55, 70, 85, and 100°C. Cells were stopped at different cycle numbers to measure gas evolution and charge transfer impedance (Rct) and then disassembled to track VC consumption, Fe deposition on the negative electrode, and diethyl-2,5-dioxahexane carboxylate (DEOHC) generation inside the cell. Higher concentrations of VC improves capacity retention, suppresses the generation of DEOHC and the rapid deposition of Fe on the negative electrode but is accompanied by more rapid gas evolution and Rct increase. Once all of the VC is consumed VC containing cells, Fe deposition accelerates and DEOHC appears in the electrolyte. These findings verify previous suggestions that lithium alkoxide accelerates Fe dissolution and the subsequent Fe deposition on the negative electrode of LFP/Graphite cells.
Sharad S Upadhyay et al 2025 J. Electrochem. Soc.
We report for the first time the application of intrinsic chirality of RRRR (+) - (18-Crown-6) - 2, 3, 11, 12- tetra carboxylic acid ((+) TCA) along with graphene nanosheets (GN) in a chiral carbon paste electrode (CCPE) system for the voltammetric discrimination of tryptophan (TRY) isomers. Embedding (+) TCA in GN enhances rigidity and forms tripodal hydrogen bonds with amine groups of L-TRY and D-TRY, providing the sensor high stereoselectivity. Experimental and quantum chemical computational studies confirmed the stronger affinity of (+) TCA for L-TRY over D-TRY using the differential pulse voltammetry (DPV) and the counterpoise method. The stability constants (1:1 complex) of L-TRY and D-TRY with (+) TCA were 1191 M⁻¹ and 588 M⁻¹, respectively. Additionally, the peak potential difference (ΔEp = EL – ED) between enantiomers on CCPE was 129 mV. The lowest energy conformation of (+) TCA-L-TRY has a Gibbs free energy higher than (+) TCA-D-TRY by 7.08 kcal/mol (B3LYP) or 9.10 kcal/mol (M06-2X), indicating a preference for the L enantiomer. The detection limits for L-TRY and D-TRY are 0.023 µM and 0.075 µM, respectively, with a linear range from 1 µM to 120 µM. The sensor quantified TRY in blood serum and urine samples.
Allan Lebreton et al 2025 J. Electrochem. Soc. 172 040523
This study delves into the impact of substrate bias voltage on vanadium nitride thin films deposited via DC magnetron sputtering. By increasing the substrate bias voltage from 0 to −200 V, the microstructure of the films changes from crystalline and porous to amorphous and dense. This obvious change in microstructure is due to atomic peening effect, which is the sputtering of the film by energetic cations at high bias voltage during thin film growth. For capacitive storage devices, it is known that the microstructure of the electrode is key to achieve high capacitance, with the aim to maximize the electrode surface area and concomitantly the areal capacitance, while keeping a low characteristic time to achieve fast charge/discharge rates. In this study, we reveal that a trade-off must be found between areal capacitance and characteristic time. Samples sputtered with low substrate bias voltages present higher areal capacitance but also higher characteristic time compared to thin films sputtered with high substrate bias voltages. The evolution of the characteristic time associated with fast charge/discharge aligns with the electrical conductivity of VN films as determined by four-point probes measurement and indicates that the cycling rate is limited by electrical properties of the VN film.
Yating Yuan et al 2025 J. Electrochem. Soc. 172 043508
The development of reliable reference electrodes is critical for advancing electrochemical studies in high-temperature molten salts. In this work, the MgMn2O4/Mg2MnO4 redox couple is utilized as a solid-state reference electrode in the ternary molten salt system MgCl2-NaCl-KCl. The electrochemical behavior of the MgMn2O4/Mg2MnO4 redox couple was evaluated via cyclic voltammetry and chronopotentiometry, which revealed a reversible insertion and extraction of Mg in MgMn2O4. The MgMn2O4 electrode displayed a stable value for 100 h when tested by monitoring the open circuit potential. The electrochemical behavior of Sb2O3 was studied in MgCl2-NaCl-KCl to demonstrate the practical utility of the electrode. Cyclic voltammetry measurements, chronoamperometry, and powder X-ray diffraction analysis confirmed the complete reduction of Sb2O3 to Sb. These findings show that the MgMn2O4/Mg2MnO4 system is a promising candidate for stable and reproducible reference electrode for applications in high-temperature molten salt systems.
Matthew Chagnot et al 2025 J. Electrochem. Soc.
The original publication acknowledged the incorrect NSF grant number for this work. The following is the correct NSF grant acknowledgement: 
This review is based upon work supported by the National Science Foundation under grant DMR-1653827.
Harrison Mar et al 2025 J. Electrochem. Soc. 172 044510
Anion-exchange membrane water electrolyzers (AEM-WE) have been identified as a promising solution to deliver green hydrogen at a lower cost than alkaline water electrolyzers (AWEs) and proton-exchange membrane water electrolyzers (PEM-WEs). However, scaling AEM-WE is limited by high voltage degradation rates which can become amplified and more complicated during operation as components within the membrane electrode assembly (MEA) evolve and interact with one another. These phenomena necessitate testing protocols that capture the degradation of individual MEA components in situ. Herein, an edge-type reference electrode and a novel flow plate design enabled decoupling of anode and cathode degradation over stability tests >200 h. A critical assessment of the overpotential measurements is provided, utilizing half-cell impedance measurements to highlight the effects of electrode misalignment. 3-electrode cyclic voltammetry is presented as an effective in situ tool to evaluate electrode degradation. These findings demonstrate the utility of edge-type reference electrode configurations in stability tests for the development of commercial scale AEM-WE.
Maik Stamm et al 2025 J. Electrochem. Soc. 172 040522
Optimization of the formation process can be a major driver for cost reduction in lithium ion battery cell production. To further tune the formation process, a deeper understanding of the effect of the voltage window during operation is required. In this regard, formation processes based on micro-cycles, i.e. cycles within different voltage windows, were used with NMC811||graphite and NM811||graphite/10%SiOx 1 Ah pouch cells. Three different micro-cycle formation voltage windows were investigated: 3.0 V–3.5 V (P1), 3.6 V–3.7 V (P2) and 4.0 V–4.2 V (P3). The P1 formation process achieved on average 3% more discharge capacity after 300 cycles for the NMC811||graphite based cells. For the SiOx containing cells, a large voltage window (P3) formation process is the least suitable, leading to an average of 4% lower discharge capacity after 300 cycles. These differences in discharge capacity were attributable to capacity loss within the formation and the initial aging cycle, rather than capacity retention. Furthermore, gas evolution and VC electrolyte additive consumption during the formation process were notably lower for the SiOx containing cells.
Katherine Betts et al 2025 J. Electrochem. Soc. 172 040521
An in situ double probe beam deflection (PBD) technique has been developed using two laser beams to map the concentration profile of the diffusion layer in an electrochemical cell. A microscale moving upper probe and a fixed position secondary beam offer real-time concentration gradients to be profiled throughout the depth of the diffusion layer. The double PBD technique was used to plot concentration profiles for 0.1 mol kg−1 CuSO4 and ZnSO4 within a range of applied currents, showing increased magnitudes of gradients for higher currents. Both single and double beam PBD were explored, demonstrating the distance and time dependence of the developing concentration gradient. While CuSO4 showed a systematic trend of increased response delay and decreased deflection with increased distance from the electrode, ZnSO4 experienced some additional phenomena affecting the refractive index within the diffusion layer. The in situ double probe beam deflection was shown to be highly sensitive and offers future work in quantifying charge migration within this important region of the electrochemical cell.