This year, the battery industry celebrates the 25th anniversary of the introduction of the lithium ion rechargeable battery by Sony Corporation. The discovery of the system dates back to earlier work by Asahi Kasei in Japan, which used a combination of lower temperature carbons for the negative electrode to prevent solvent degradation and lithium cobalt dioxide modified somewhat from Goodenough's earlier work. The development by Sony was carried out within a few years by bringing together technology in film coating from their magnetic tape division and electrochemical technology from their battery division. The past 25 years has shown rapid growth in the sales and in the benefits of lithium ion in comparison to all the earlier rechargeable battery systems. Recent work on new materials shows that there is a good likelihood that the lithium ion battery will continue to improve in cost, energy, safety and power capability and will be a formidable competitor for some years to come.

The Electrochemical Society (ECS) was founded in 1902 to advance the theory and practice at the forefront of electrochemical and solid state science and technology, and allied subjects.
ISSN: 1945-7111
JES is the flagship journal of The Electrochemical Society. Published continuously from 1902 to the present, JES remains one of the most highly-cited journals in electrochemistry and solid-state science and technology.
George E. Blomgren 2017 J. Electrochem. Soc. 164 A5019
Manuel Ank et al 2023 J. Electrochem. Soc. 170 120536
Battery research depends upon up-to-date information on the cell characteristics found in current electric vehicles, which is exacerbated by the deployment of novel formats and architectures. This necessitates open access to cell characterization data. Therefore, this study examines the architecture and performance of first-generation Tesla 4680 cells in detail, both by electrical characterization and thermal investigations at cell-level and by disassembling one cell down to the material level including a three-electrode analysis. The cell teardown reveals the complex cell architecture with electrode disks of hexagonal symmetry as well as an electrode winding consisting of a double-sided and homogeneously coated cathode and anode, two separators and no mandrel. A solvent-free anode fabrication and coating process can be derived. Energy-dispersive X-ray spectroscopy as well as differential voltage, incremental capacity and three-electrode analysis confirm a NMC811 cathode and a pure graphite anode without silicon. On cell-level, energy densities of 622.4 Wh/L and 232.5 Wh/kg were determined while characteristic state-of-charge dependencies regarding resistance and impedance behavior are revealed using hybrid pulse power characterization and electrochemical impedance spectroscopy. A comparatively high surface temperature of ∼70 °C is observed when charging at 2C without active cooling. All measurement data of this characterization study are provided as open source.
Eniko S. Zsoldos et al 2024 J. Electrochem. Soc. 171 080527
Lithium iron phosphate (LFP) battery cells are ubiquitous in electric vehicles and stationary energy storage because they are cheap and have a long lifetime. This work compares LFP/graphite pouch cells undergoing charge-discharge cycles over five state of charge (SOC) windows (0%–25%, 0%–60%, 0%–80%, 0%–100%, and 75%–100%). Cycling LFP cells across a lower average SOC results in less capacity fade than cycling across a higher average SOC, regardless of depth of discharge. The primary capacity fade mechanism is lithium inventory loss due to: lithiated graphite reactivity with electrolyte, which increases incrementally with SOC, and lithium alkoxide species causing iron dissolution and deposition on the negative electrode at high SOC which further accelerates lithium inventory loss. Our results show that even low voltage LFP systems (3.65 V) have a tradeoff between average SOC and lifetime. Operating LFP cells at lower average SOC can extend their lifetime substantially in both EV and grid storage applications.
Yuliya Preger et al 2020 J. Electrochem. Soc. 167 120532
Energy storage systems with Li-ion batteries are increasingly deployed to maintain a robust and resilient grid and facilitate the integration of renewable energy resources. However, appropriate selection of cells for different applications is difficult due to limited public data comparing the most commonly used off-the-shelf Li-ion chemistries under the same operating conditions. This article details a multi-year cycling study of commercial LiFePO4 (LFP), LiNixCoyAl1−x−yO2 (NCA), and LiNixMnyCo1−x−yO2 (NMC) cells, varying the discharge rate, depth of discharge (DOD), and environment temperature. The capacity and discharge energy retention, as well as the round-trip efficiency, were compared. Even when operated within manufacturer specifications, the range of cycling conditions had a profound effect on cell degradation, with time to reach 80% capacity varying by thousands of hours and cycle counts among cells of each chemistry. The degradation of cells in this study was compared to that of similar cells in previous studies to identify universal trends and to provide a standard deviation for performance. All cycling files have been made publicly available at batteryarchive.org, a recently developed repository for visualization and comparison of battery data, to facilitate future experimental and modeling efforts.
Peter Keil et al 2016 J. Electrochem. Soc. 163 A1872
In this study, the calendar aging of lithium-ion batteries is investigated at different temperatures for 16 states of charge (SoCs) from 0 to 100%. Three types of 18650 lithium-ion cells, containing different cathode materials, have been examined. Our study demonstrates that calendar aging does not increase steadily with the SoC. Instead, plateau regions, covering SoC intervals of more than 20%–30% of the cell capacity, are observed wherein the capacity fade is similar. Differential voltage analyses confirm that the capacity fade is mainly caused by a shift in the electrode balancing. Furthermore, our study reveals the high impact of the graphite electrode on calendar aging. Lower anode potentials, which aggravate electrolyte reduction and thus promote solid electrolyte interphase growth, have been identified as the main driver of capacity fade during storage. In the high SoC regime where the graphite anode is lithiated more than 50%, the low anode potential accelerates the loss of cyclable lithium, which in turn distorts the electrode balancing. Aging mechanisms induced by high cell potential, such as electrolyte oxidation or transition-metal dissolution, seem to play only a minor role. To maximize battery life, high storage SoCs corresponding to low anode potential should be avoided.
Chang-Hui Chen et al 2020 J. Electrochem. Soc. 167 080534
Presented here, is an extensive 35 parameter experimental data set of a cylindrical 21700 commercial cell (LGM50), for an electrochemical pseudo-two-dimensional (P2D) model. The experimental methodologies for tear-down and subsequent chemical, physical, electrochemical kinetics and thermodynamic analysis, and their accuracy and validity are discussed. Chemical analysis of the LGM50 cell shows that it is comprised of a NMC 811 positive electrode and bi-component Graphite-SiOx negative electrode. The thermodynamic open circuit voltages (OCV) and lithium stoichiometry in the electrode are obtained using galvanostatic intermittent titration technique (GITT) in half cell and three-electrode full cell configurations. The activation energy and exchange current coefficient through electrochemical impedance spectroscopy (EIS) measurements. Apparent diffusion coefficients are estimated using the Sand equation on the voltage transient during the current pulse; an expansion factor was applied to the bi-component negative electrode data to reflect the average change in effective surface area during lithiation. The 35 parameters are applied within a P2D model to show the fit to experimental validation LGM50 cell discharge and relaxation voltage profiles at room temperature. The accuracy and validity of the processes and the techniques in the determination of these parameters are discussed, including opportunities for further modelling and data analysis improvements.
Anar Badalbayli et al 2025 J. Electrochem. Soc. 172 032508
This study explores chloride molten salt electrolysis (CMSE) as a promising route for energy-efficient iron metal (Fe) production. Moderate temperature (500 °C) LiCl-KCl molten salts offer excellent thermodynamic stability, high ionic conductivity and diffusivity, and high solubility for FeCl3, thereby enabling efficient Fe metal extraction at high electrowinning rates. Here, we demonstrate the two essential steps for converting taconite ore into Fe metal. First, Fe2O3 from taconite pellets was selectively leached in HCl yielding a high-purity FeCl3 aqueous solution, while the gangue components settled at the bottom. Then, anhydrous FeCl3 was electrolyzed in a LiCl-KCl eutectic molten salt at 500 °C at high current density (1 A cm−2) and at high Coulombic efficiency (>85%). Analysis of the electrowon Fe deposits revealed dendritic structures with purity of >99 wt%, which could be further improved to nearly 100 wt% through arc re-melting. CMSE offers low specific energy consumption (3.7 kWhr kg−1), competitive with H2-DRI and other electrolytic approaches being pursued globally. Our findings underscore the potential of CMSE as an energy-efficient route for electrosynthesis of Fe metal.
E. Peled and S. Menkin 2017 J. Electrochem. Soc. 164 A1703
The Solid-Electrolyte-Interphase (SEI) model for non-aqueous alkali-metal batteries constitutes a paradigm change in the understanding of lithium batteries and has thus enabled the development of safer, durable, higher-power and lower-cost lithium batteries for portable and EV applications. Prior to the publication of the SEI model (1979), researchers used the Butler-Volmer equation, in which a direct electron transfer from the electrode to lithium cations in the solution is assumed. The SEI model proved that this is a mistaken concept and that, in practice, the transfer of electrons from the electrode to the solution in a lithium battery, must be prevented, since it will result in fast self-discharge of the active materials and poor battery performance. This model provides [E. Peled, in "Lithium Batteries," J.P. Gabano (ed), Academic Press, (1983), E. Peled, J. Electrochem. Soc., 126, 2047 (1979).] new equations for: electrode kinetics (io and b), anode corrosion, SEI resistivity and growth rate and irreversible capacity loss of lithium-ion batteries. This model became a cornerstone in the science and technology of lithium batteries. This paper reviews the past, present and the future of SEI batteries.
Roland Jung et al 2017 J. Electrochem. Soc. 164 A1361
Layered LiNixMnyCozO2 (NMC) is a widely used class of cathode materials with LiNi1/3Mn1/3Co1/3O2 (NMC111) being the most common representative. However, Ni-rich NMCs are more and more in the focus of current research due to their higher specific capacity and energy. In this work we will compare LiNi1/3Mn1/3Co1/3O2 (NMC111), LiNi0.6Mn0.2Co0.2O2 (NMC622), and LiNi0.8Mn0.1Co0.1O2 (NMC811) with respect to their cycling stability in NMC-graphite full-cells at different end-of-charge potentials. It will be shown that stable cycling is possible up to 4.4 V for NMC111 and NMC622 and only up to 4.0 V for NMC811. At higher potentials, significant capacity fading was observed, which was traced back to an increase in the polarization of the NMC electrode, contrary to the nearly constant polarization of the graphite electrode. Furthermore, we show that the increase in the polarization occurs when the NMC materials are cycled up to a high-voltage feature in the dq/dV plot, which occurs at ∼4.7 V vs. Li/Li+ for NMC111 and NMC622 and at ∼4.3 V vs. Li/Li+ for NMC811. For the latter material, this feature corresponds to the H2 → H3 phase transition. Contrary to the common understanding that the electrochemical oxidation of carbonate electrolytes causes the CO2 and CO evolution at potentials above 4.7 V vs. Li/Li+, we believe that the observed CO2 and CO are mainly due to the chemical reaction of reactive lattice oxygen with the electrolyte. This hypothesis is based on gas analysis using On-line Electrochemical Mass Spectrometry (OEMS), by which we prove that all three materials release oxygen from the particle surface and that the oxygen evolution coincides with the onset of CO2 and CO evolution. Interestingly, the onsets of oxygen evolution for the different NMCs correlate well with the high-voltage redox feature at ∼4.7 V vs. Li/Li+ for NMC111 and NMC622 as well as at ∼4.3 V vs. Li/Li+ for NMC811. To support this hypothesis, we show that no CO2 or CO is evolved for the LiNi0.43Mn1.57O4 (LNMO) spinel up to 5 V vs. Li/Li+, consistent with the absence of oxygen release. Lastly, we demonstrate by the use of 13C labeled conductive carbon that it is the electrolyte rather than the conductive carbon which is oxidized by the released lattice oxygen. Taking these findings into consideration, a mechanism is proposed for the reaction of released lattice oxygen with ethylene carbonate yielding CO2, CO, and H2O.
A. K. Padhi et al 1997 J. Electrochem. Soc. 144 1188
Reversible extraction of lithium from 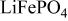 (triphylite) and insertion of lithium into
(triphylite) and insertion of lithium into 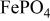 at 3.5 V vs. lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low‐power, rechargeable lithium battery that is inexpensive, nontoxic, and environmentally benign. Electrochemical extraction was limited to ∼0.6 Li/formula unit; but even with this restriction the specific capacity is 100 to 110 mAh/g. Complete extraction of lithium was performed chemically; it gave a new phase,
at 3.5 V vs. lithium at 0.05 mA/cm2 shows this material to be an excellent candidate for the cathode of a low‐power, rechargeable lithium battery that is inexpensive, nontoxic, and environmentally benign. Electrochemical extraction was limited to ∼0.6 Li/formula unit; but even with this restriction the specific capacity is 100 to 110 mAh/g. Complete extraction of lithium was performed chemically; it gave a new phase, 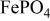 , isostructural with heterosite,
, isostructural with heterosite, 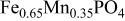 . The
. The 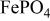 framework of the ordered olivine
framework of the ordered olivine 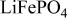 is retained with minor displacive adjustments. Nevertheless the insertion/extraction reaction proceeds via a two‐phase process, and a reversible loss in capacity with increasing current density appears to be associated with a diffusion‐limited transfer of lithium across the two‐phase interface. Electrochemical extraction of lithium from isostructural
is retained with minor displacive adjustments. Nevertheless the insertion/extraction reaction proceeds via a two‐phase process, and a reversible loss in capacity with increasing current density appears to be associated with a diffusion‐limited transfer of lithium across the two‐phase interface. Electrochemical extraction of lithium from isostructural 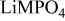 (M = Mn, Co, or Ni) with an
(M = Mn, Co, or Ni) with an 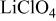 electrolyte was not possible; but successful extraction of lithium from
electrolyte was not possible; but successful extraction of lithium from 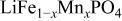 was accomplished with maximum oxidation of the
was accomplished with maximum oxidation of the 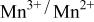 occurring at x = 0.5. The
occurring at x = 0.5. The 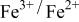 couple was oxidized first at 3.5 V followed by oxidation of the
couple was oxidized first at 3.5 V followed by oxidation of the 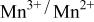 couple at 4.1 V vs. lithium. The
couple at 4.1 V vs. lithium. The 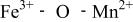 interactions appear to destabilize the
interactions appear to destabilize the 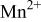 level and stabilize the
level and stabilize the 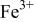 level so as to make the
level so as to make the 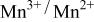 energy accessible.
energy accessible.
Maria Kelly et al 2025 J. Electrochem. Soc. 172 046503
Voltammetric measurements of electrochemical CO2 reduction reaction (CO2RR) selectivity on rotating ring disk electrodes (RRDE) are a rapid and sensitive method for quantifying an electrocatalyst's selectivity, i.e. faradaic efficiency (FE). This method has been applied to polycrystalline Au electrocatalysts where a Au disk electrode catalyzes both the CO2RR and hydrogen evolution reaction while the concentric Au ring electrode selectively senses CO by oxidizing CO back to CO2. Such measurements enabled fundamental mechanistic studies but suffer from poor inter-laboratory reproducibility. This work identifies causes of variability in RRDE selectivity measurements by comparing protocols with different electrochemical methods, reagent purities, and glassware cleaning procedures. We observed FECO decrease by 56% during 5 min chronoamperometry measurements, a phenomenon that is not readily apparent in voltammetric scans due to their dynamic nature. Electroplating of electrolyte impurities onto the disk and ring surfaces were identified as a major contributor to Au deactivation. Additionally, the oxygen reduction reaction may lead to higher disk currents in inadequately purged electrolytes, causing an apparent underestimation of FECO at low overpotentials. Lastly, we propose operational bounds for CO2RR selectivity measurements on Au using the RRDE method and provide suggestions on steps for improving the accuracy of this technique.
figure placeholder
Heather Hamilton and Charles L. Hussey 2025 J. Electrochem. Soc. 172 046502
Electrochemical measurements were used to probe the solvation by chloride and the accessible oxidation states of Dy, Er, Gd, Ho, Tb, and Tm in a room-temperature ionic liquid (RTIL) based on the 1-ethyl-3-methylimidazolium cation (EtMeIm+) and the bis(trifluoromethylsulfonyl)amide anion (Tf2N−). Controlled potential coulometry indicated that all these lanthanide (Ln) metals were oxidized into the RTILs as the corresponding Ln3+ species. Amperometric titration experiments with added Cl− revealed that this anion coordinates with each Ln3+ to form a likely octahedral complex represented as [LnCl6]3−. Of the Ln3+ solutions that were prepared by CPC, cyclic staircase voltammetry indicated that only Tm3+ exhibited electrochemical activity within the potential window of the solvent. Thus, we report here the first electrochemical investigation of the Tm3+/2+ reaction in an anhydrous low melting ionic liquid. The reduction of Tm3+ to Tm2+ is complicated by the disproportionation of the Tm2+ to Tm3+ and Tm0. The latter appears to react with the ionic liquid cation.
Ruiwei Cui et al 2025 J. Electrochem. Soc. 172 044508
Based on the acid etching strategy proposed in 2022, a novel treating method is developed and employed in the Sm0.2Ce0.8O2−δ (SDC) based SOFC. After 25 min acid treatment, the surface roughness of SDC increases by 60% while the peeling strength of cathode/electrolyte interface can be enhanced by 129%. The peak power density at 923 K reaches 1.392 W·cm−2, 30% higher than that (1.067 W·cm−2) without treatment. Compared with little degradation observed during working at 873 K for 200 h, apparent performance degradation of single cells can be observed during thermal cycling at 673–873 K. However, the thermal cycling stability can be markedly improved by the 25 min acid etch. Combined with the electrochemical impedance spectroscopy and microstructure observation, this suggests that the significantly enhanced performance should be caused by the synergy effect of both revitalized interface and increased bonding area between cathodes and electrolytes.
figure placeholder
Highlights
Acid etch of electrolyte significantly promotes the interface bonding.
The highest PPD at 923 K (1.392 W·cm−2) can be obtained by 25 min acid etching.
The thermal cycling stability can be apparently enhanced by acid etching.
Improved performance should be caused by the increased bonding area.
Mengyao Zhang et al 2025 J. Electrochem. Soc. 172 040513
The sodium super-ionic conductor (NaSICON) has versatile applications as a ceramic electrolyte for energy storage, where it can serve as an impermeable separator in solid-state batteries and redox flow systems. In particular, NaSICON systems have been proposed to be relatively stable in contact with water, making them compatible with aqueous battery chemistries. However, owing to their brittle nature and metal oxide constituents, stress-corrosion cracking (SCC) is an important failure mechanism that has not been previously explored. In this study, we assess the fracture toughness of NaSICON membranes in contact with aqueous solutions that are relevant to redox flow systems. Microindentation was performed to generate visible surface cracks and residual stress, which were observed to grow in length after exposure to aqueous solutions. This allows for a quantitative measurement of fracture toughness, which decreases after exposure to water. To contextualize these results, we develop a simplified model of the fracture behavior in aqueous redox-flow batteries that incorporate NaSICON membranes, illustrating the importance of SCC in cell design. This work provides quantitative insights into SCC as a failure mode in NaSICON, enhancing our understanding of the chemo-mechanical behavior of ceramic electrolytes in contact with aqueous solutions.
Xuefei Zhang et al 2025 J. Electrochem. Soc. 172 043505
Electrocatalytic oxidation using PbO2 electrodes is a prospective strategy for treatment of bio-refractory dye wastewater. However, it is hindered by high energy consumption, poor degradation efficiency, and low anode stability. Here, PbO2 electrodes were reconstructed by intercalating and surface-intervening with graphene oxide (GO) to enhance the stability of the composite electrodes and achieve high-efficiency oxidation of cationic red GTL. Compared with that on PbO2, reactive oxygen species (e.g., ·OH and ·O) on GO-PbO2 were less consumed by the oxygen evolution reaction due to their larger spacing on GO-PbO2. In addition, GO-PbO2 showed lower cell voltage than PbO2, indicating a higher electron transfer rate and lower energy consumption. The coupled free radical behavior and electron transfer enhanced the decolorization (1.7 times that of PbO2) and mineralization (1.4 times that of PbO2) rate of cationic red GTL on GO-PbO2. Furthermore, doping of GO with multilayer structure improved the stability of PbO2 anode and its performance improvement mechanism was revealed. The prepared GO-PbO2 electrode prolonged service-life (162 h) under accelerated electrolysis, about 2.7 times that of the PbO2 electrode (60 h). This work provides a novel GO-PbO2 electrode for efficient electrocatalytic degradation of GTL, and can be easily extended to electro-degradation of non-biodegradable wastewater.
Shao-bang Pan et al 2025 J. Electrochem. Soc. 172 044507
As a promising low-cost solar energy conversion technology, dye-sensitized solar cells have attracted widespread attention from researchers due to their low cost, simple preparation method, low toxicity, and easy production. Improving the functional conversion efficiency of batteries has always been an important research direction in this field to obtain more commercial application prospects. Compared to a single photosensitive molecule, the combined action of multiple photosensitizers sometimes exhibits superior photovoltaic performance. In order to provide people with a better understanding of the extraordinary changes brought about by co-sensitization, this article introduces the research results of co-sensitization of various photosensitive dyes with N719 in the past 10 years from different perspectives according to the types of anchoring groups (cyanoacrylic acid, carboxyl group, rhodanine acetic acid, pyridine, and cyanoethylene benzoic acid), hoping to assist with further research on co sensitization mechanisms in the future.
Rongcen Zhao et al 2025 J. Electrochem. Soc. 172 034512
Electrolysis serves as an effective technique for metal preparation, with the electrolytic cell being the fundamental apparatus. The design of the electrolytic cell significantly influences the mass transfer process. Therefore, it is crucial to create an appropriate structure for the electrolytic cell to minimize energy consumption during electrolysis. Given the unique characteristics of the metals involved, the configurations of electrolytic cells vary accordingly. This article examines primary metals produced through electrolysis, such as aluminum and alkali metals, and discusses advancements in research and design principles related to electrolytic cell structures. It also compares various types of electrolytic cells and suggests strategies for structural optimization. Additionally, the role of simulation in the design of electrolytic cells is emphasized. Finally, the article addresses the challenges encountered by electrolytic cells in industrial settings and offers recommendations for structural improvements.
figure placeholder
İsmail Mert Vural and Nurgul K. Bakirhan 2025 J. Electrochem. Soc. 172 037508
The opioid crisis has emerged as a critical public health issue, characterized by the widespread misuse, addiction, and adverse societal impacts of opioid substances. Addressing this multifaceted crisis demands innovative approaches, and the field of forensic science has increasingly turned to electrochemical methods as a powerful tool in the battle against opioids. Here we provide an overview of the significant role played by electrochemical techniques in the detection, analysis, and monitoring of opioids. By harnessing the capabilities of electrochemical sensors, nanomaterial-based platforms, and microfluidic devices, forensic scientists have achieved breakthroughs in opioid detection, offering higher sensitivity, specificity, and rapidity than traditional methods. We explore the latest advancements and applications of electrochemical techniques in forensic opioid analysis, highlighting their potential to revolutionize not only the investigative process but also the management of opioid-related crises. With an emphasis on real-time, on-site, and non-invasive detection, we underscore the importance of electrochemical techniques as a vital component in combating the opioid epidemic and contributing to public safety and well-being.
Alfred B. Anderson 2025 J. Electrochem. Soc. 172 036501
For many years since Gurney introduced quantum mechanics to electrochemistry, models and calculations assumed bonding and other properties at the electrochemical interface may be calculated with adequate accuracy at the potential of zero charge (PZC) and that the effect of potential lies solely in controlling the energy of the electron involved in the transfer, which comes from or goes to an external energy level. The energy of the electron is assigned to the Fermi energy, Ef, of the electrode for the particular potential being modeled. This is done in the Butler-Volmer theory as well as in several quantum mechanical modeling procedures that are introduced here. Though the PZC in fact changes as the identity, amount, and structures of molecules chemically bonded to the electrode are varied during calculations using these models, there is no control of the electrode potential in the calculations. The past two decades have seen the development of computer codes that can incorporate controlled incremental surface charging with polarizable electrolyte models that compensate it, resulting in zero net interface charge. Calculations using these codes provide accurate predictions of the potential-dependent energies of reactants and products, reversible potentials, and electron transfer activation energies.
Yang Xiao et al 2025 J. Electrochem. Soc. 172 034504
Due to the advantages of environmental friendliness and high energy density, fuel cells have broad application prospects in many fields, such as automobiles, ships, aerospace, etc However, commercial applications of fuel cells also face challenges of durability and reliability, especially in shock and vibration environments. Here, the electrochemical and mechanical behaviours of fuel cells under vibration environments are described, and the effects of vibration and shock conditions on the electrochemical, mechanical, water and gas transport, and durability performance of fuel cells are systematically reviewed, involving the variation laws of assembly torque, sealing, relative slippage between cells, water and gas transport, electrical resistance, and membrane electrodes. In addition, the methods that can mitigate the effects of vibration on fuel cells in existing studies are summarised. Finally, discussions and perspectives on the research methods of fuel cell performance under vibration are presented. It is hoped that the review can provide a systematic comprehension and direction for vibration protection of fuel cells.
figure placeholder
Highlights
Vibration and shock have a negative impact on fuel cell performance in most cases.
Fuel cell performance degradation is affected by the coupling of multiple phenomena.
Specific vibration levels improve cell performance by facilitating water management.
Monitoring and vibration isolation/damping enable vibration protection of fuel cells.
Maria Kelly et al 2025 J. Electrochem. Soc. 172 046503
Voltammetric measurements of electrochemical CO2 reduction reaction (CO2RR) selectivity on rotating ring disk electrodes (RRDE) are a rapid and sensitive method for quantifying an electrocatalyst's selectivity, i.e. faradaic efficiency (FE). This method has been applied to polycrystalline Au electrocatalysts where a Au disk electrode catalyzes both the CO2RR and hydrogen evolution reaction while the concentric Au ring electrode selectively senses CO by oxidizing CO back to CO2. Such measurements enabled fundamental mechanistic studies but suffer from poor inter-laboratory reproducibility. This work identifies causes of variability in RRDE selectivity measurements by comparing protocols with different electrochemical methods, reagent purities, and glassware cleaning procedures. We observed FECO decrease by 56% during 5 min chronoamperometry measurements, a phenomenon that is not readily apparent in voltammetric scans due to their dynamic nature. Electroplating of electrolyte impurities onto the disk and ring surfaces were identified as a major contributor to Au deactivation. Additionally, the oxygen reduction reaction may lead to higher disk currents in inadequately purged electrolytes, causing an apparent underestimation of FECO at low overpotentials. Lastly, we propose operational bounds for CO2RR selectivity measurements on Au using the RRDE method and provide suggestions on steps for improving the accuracy of this technique.
figure placeholder
Anar Badalbayli et al 2025 J. Electrochem. Soc. 172 032508
This study explores chloride molten salt electrolysis (CMSE) as a promising route for energy-efficient iron metal (Fe) production. Moderate temperature (500 °C) LiCl-KCl molten salts offer excellent thermodynamic stability, high ionic conductivity and diffusivity, and high solubility for FeCl3, thereby enabling efficient Fe metal extraction at high electrowinning rates. Here, we demonstrate the two essential steps for converting taconite ore into Fe metal. First, Fe2O3 from taconite pellets was selectively leached in HCl yielding a high-purity FeCl3 aqueous solution, while the gangue components settled at the bottom. Then, anhydrous FeCl3 was electrolyzed in a LiCl-KCl eutectic molten salt at 500 °C at high current density (1 A cm−2) and at high Coulombic efficiency (>85%). Analysis of the electrowon Fe deposits revealed dendritic structures with purity of >99 wt%, which could be further improved to nearly 100 wt% through arc re-melting. CMSE offers low specific energy consumption (3.7 kWhr kg−1), competitive with H2-DRI and other electrolytic approaches being pursued globally. Our findings underscore the potential of CMSE as an energy-efficient route for electrosynthesis of Fe metal.
Guoxin Li et al 2025 J. Electrochem. Soc. 172 026501
Cathode-electrolyte interphase (CEI) is critical for inhibiting the cathode degradation to maintain cell life. However, the evolution of the CEI is still unclear due to its complex and slow dynamic process. Here we used scanning electrochemical microscopy (SECM) for in situ investigation of CEI formation process on LiFePO4 cathode. Feedback images and probe scan curves showed a heterogeneous passivation that was gently generated on the LiFePO4 particles during both charging and discharging. Besides, a LiFePO4 composited electrode was also used to investigate the CEI formation to simulate the condition of real battery system. The composited cathode does not show obvious CEI formation within first two cycles. The SECM results between the pristine LiFePO4 particles and the composited LiFePO4 indicated the dynamic accumulation of CEI, which is influenced by the ability to charge transfer kinetics of cathode materials. This approach provided a feasible consideration for the connections between the dynamic evolution of the CEI and changes in charge transfer capability of cathode during cycling.
figure placeholder
Highlights
In-situ investigation of cathode-electrolyte interphase formation.
The evolution of native active material and composite slurry were compared.
The electrochemical activity change upon cathode cycling are analysed in situ.
The influence of the charge transfer capability upon CEI generation is revealed.
D. Noel Buckley and Johna Leddy 2024 J. Electrochem. Soc. 171 116503
We revisit the classical derivation of the Butler-Volmer equation to include the effect of the electrode metal. If the metal is replaced by one with a different work function, keeping other conditions in the electrode constant, the chemical potential of electrons  and the Galvani potential
and the Galvani potential  change in a complementary manner. Changes in
change in a complementary manner. Changes in  and
and  each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of
each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of  Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both
Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both  and
and  The exchange current density
The exchange current density  appears as an exponential function of
appears as an exponential function of  For the work function
For the work function  of the metal, the approximation
of the metal, the approximation  yields a linear relationship between
yields a linear relationship between  and
and  The linear increase in
The linear increase in  with
with  has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
figure placeholder
Philip Minnmann et al 2024 J. Electrochem. Soc. 171 060514
The kinetics of composite cathodes for solid-state batteries (SSBs) relies heavily on their microstructure. Spatial distribution of the different phases, porosity, interface areas, and tortuosity factors are important descriptors that need accurate quantification for models to predict the electrochemistry and mechanics of SSBs. In this study, high-resolution focused ion beam-scanning electron microscopy tomography was used to investigate the microstructure of cathodes composed of a nickel-rich cathode active material (NCM) and a thiophosphate-based inorganic solid electrolyte (ISE). The influence of the ISE particle size on the microstructure of the cathode was visualized by 3D reconstruction and charge transport simulation. By comparison of experimentally determined and simulated conductivities of composite cathodes with different ISE particle sizes, the electrode charge transport kinetics is evaluated. Porosity is shown to have a major influence on the cell kinetics and the evaluation of the active mass of electrochemically active particles reveals a higher fraction of connected NCM particles in electrode composites utilizing smaller ISE particles. The results highlight the importance of homogeneous and optimized microstructures for high performance SSBs, securing fast ion and electron transport.
Garrick et al
In this work we demonstrate the applicability of a versatile separator-reference mounted electrode in a three-electrode setup to accurately capture the primary current pathway in the battery cell during operation, calibrate a porous electrode model to the individual anode and cathode potential signals, and assign the various resistances to electrochemical phenomena in the battery cell. This calibrated electrochemical model is validated using continuous rate discharges associated with highway driving scenarios in an electric vehicle, and in turn utilized to predict the local anode potential and proximity to lithium plating onset. Finally, we demonstrate the strategy associated with utilization of the model to estimate constant anode potential charging during fast charge scenarios at various rates and starting conditions as a future look to fast charge calibration development and controls.
Betts et al
An in situ double probe beam deflection (PBD) technique has been developed using two laser beams to map the concentration profile of the diffusion layer in an electrochemical cell. A microscale moving upper probe and a fixed position secondary beam offer real-time concentration gradients to be profiled throughout the depth of the diffusion layer. The double PBD technique was used to plot concentration profiles for 0.1 mol/kg CuSO4 and ZnSO4 within a range of applied currents, showing increased magnitudes of gradients for higher currents. Both single and double beam PBD were explored, demonstrating the distance and time dependence of the developing concentration gradient. While CuSO4 showed a systematic trend of increased response delay and decreased deflection with increased distance from the electrode, ZnSO4 experienced some additional phenomena affecting the refractive index within the diffusion layer. The in situ double probe beam deflection was shown to be highly sensitive and offers future work in quantifying charge migration within this important region of the electrochemical cell.
Hickson et al
The passage of current through a battery results in the development of concentration gradients in the electrolytic phase. For a fully characterized binary electrolyte, where the conductivity, salt diffusion coefficient, cation transference number, and the thermodynamic factor are known, concentration and potential gradients in the electrolytic phase can be modeled using Newman’s concentrated solution theory. We report two methods for measuring the transference number: the standard method based on electrochemical measurements (t_(+,echem)^0) and electrophoretic NMR (t_(+,eNMR)^0). The electrochemical approach requires combining measurements from multiple experiments; the equations used to determine the cation transference number and the thermodynamic factor are coupled, nonlinear algebraic equations. In the electrophoretic-NMR-based approach, however, the equations used to determine the cation transference number and the thermodynamic factor are decoupled. We find for a liquid electrolyte comprised of a lithium salt dissolved in tetraglyme, the values of the transference numbers obtained by these two methods are distinct. For example, at 30C, t_(+,echem)^0 = -1.02±1.11 and t_(+,eNMR)^0 = 0.25±0.04. The corresponding thermodynamic factors are also different. While the magnitude of the predicted concentration gradients based on the two sets of parameters are different, the predicted current-voltage relationships are similar.
Zúñiga Martínez et al
A novel synthesis approach for Cu0.16VOPO4•2.5H2O material is reported, consisting of VOPO4 layers incorporating water molecules and Cu2+ ions within the interlayer space. The inclusion of Cu2+ ions leads to significant changes in the previously reported electrochemical properties of VOPO4•2H2O. Indeed, copper ion insertion leads to a reduced interlayer space, a higher surface area, and, consequently, to a higher specific charge. Moreover, the appearance of new fast faradaic reactions is depicted from the presence of new redox peaks in cyclic voltammograms. The capacity of this material is 93 Cg-1 in 3M LiOH, 114 Cg-1 in 3M NaOH, and 126 Cg-1 in 3M KOH at a scan rate of 5 mVs-1. It was determined that an intercalation process takes place across the entire operational range in all three electrolytes, even at scan rates as high as 500 mVs-1. Additionally, electrochemical impedance spectroscopy (EIS) was used for a more comprehensive understanding of the electrochemical role of the interlayer Cu2+ cations. EIS enables us to propose a new mechanism of electron transfer between VOPO4 layers and Cu2+ ion layers, which extends to neighboring layers, thus explaining the fast kinetic of the related faradaic reactions.
Motevalian et al
The polysulfide shuttle effect remains a fundamental challenge in lithium-sulfur batteries, particularly for high-energy-density applications where conventional mitigation strategies prove insufficient. Here, we introduce a state-resolved methodology for quantifying shuttle current by analyzing Coulombic efficiency across discretized charging blocks, addressing limitations in traditional voltage-dependent measurement techniques. Through systematic analysis of cells with and without LiNO₃ additive, we demonstrate that shuttle activity peaks at 60-70% state of charge (SOC), correlating with maximum Li₂S₄ concentration as confirmed by UV-vis spectroscopy. The block efficiency analysis reveals distinct patterns: cells without LiNO₃ show efficiency dropping to 60% in the mid-SOC region, while LiNO₃-containing cells maintain minimum efficiency around 80%, demonstrating approximately 70% suppression of peak shuttle current. Electrochemical impedance analysis further reveals how polysulfide evolution affects transport processes, with bulk resistance peaking at mid-SOC due to pore blockage, while interfacial resistance changes reflect the transition between different polysulfide species. By correlating block efficiency with polysulfide speciation, we establish that Li₂S₄ drives shuttle activity through its optimal balance of solubility and mobility, while larger Li₂S₈ species contribute less despite higher solubility. This work provides quantitative insights into shuttle current distribution across different SOC ranges while establishing a robust methodology for evaluating shuttle suppression strategies.
Maria Kelly et al 2025 J. Electrochem. Soc. 172 046503
Voltammetric measurements of electrochemical CO2 reduction reaction (CO2RR) selectivity on rotating ring disk electrodes (RRDE) are a rapid and sensitive method for quantifying an electrocatalyst’s selectivity, i.e. faradaic efficiency (FE). This method has been applied to polycrystalline Au electrocatalysts where a Au disk electrode catalyzes both the CO2RR and hydrogen evolution reaction while the concentric Au ring electrode selectively senses CO by oxidizing CO back to CO2. Such measurements enabled fundamental mechanistic studies but suffer from poor inter-laboratory reproducibility. This work identifies causes of variability in RRDE selectivity measurements by comparing protocols with different electrochemical methods, reagent purities, and glassware cleaning procedures. We observed FECO decrease by 56% during 5 min chronoamperometry measurements, a phenomenon that is not readily apparent in voltammetric scans due to their dynamic nature. Electroplating of electrolyte impurities onto the disk and ring surfaces were identified as a major contributor to Au deactivation. Additionally, the oxygen reduction reaction may lead to higher disk currents in inadequately purged electrolytes, causing an apparent underestimation of FECO at low overpotentials. Lastly, we propose operational bounds for CO2RR selectivity measurements on Au using the RRDE method and provide suggestions on steps for improving the accuracy of this technique.
figure placeholder
Heather Hamilton and Charles L. Hussey 2025 J. Electrochem. Soc. 172 046502
Electrochemical measurements were used to probe the solvation by chloride and the accessible oxidation states of Dy, Er, Gd, Ho, Tb, and Tm in a room-temperature ionic liquid (RTIL) based on the 1-ethyl-3-methylimidazolium cation (EtMeIm+) and the bis(trifluoromethylsulfonyl)amide anion (Tf2N−). Controlled potential coulometry indicated that all these lanthanide (Ln) metals were oxidized into the RTILs as the corresponding Ln3+ species. Amperometric titration experiments with added Cl− revealed that this anion coordinates with each Ln3+ to form a likely octahedral complex represented as [LnCl6]3−. Of the Ln3+ solutions that were prepared by CPC, cyclic staircase voltammetry indicated that only Tm3+ exhibited electrochemical activity within the potential window of the solvent. Thus, we report here the first electrochemical investigation of the Tm3+/2+ reaction in an anhydrous low melting ionic liquid. The reduction of Tm3+ to Tm2+ is complicated by the disproportionation of the Tm2+ to Tm3+ and Tm0. The latter appears to react with the ionic liquid cation.
Mengyao Zhang et al 2025 J. Electrochem. Soc. 172 040513
The sodium super-ionic conductor (NaSICON) has versatile applications as a ceramic electrolyte for energy storage, where it can serve as an impermeable separator in solid-state batteries and redox flow systems. In particular, NaSICON systems have been proposed to be relatively stable in contact with water, making them compatible with aqueous battery chemistries. However, owing to their brittle nature and metal oxide constituents, stress-corrosion cracking (SCC) is an important failure mechanism that has not been previously explored. In this study, we assess the fracture toughness of NaSICON membranes in contact with aqueous solutions that are relevant to redox flow systems. Microindentation was performed to generate visible surface cracks and residual stress, which were observed to grow in length after exposure to aqueous solutions. This allows for a quantitative measurement of fracture toughness, which decreases after exposure to water. To contextualize these results, we develop a simplified model of the fracture behavior in aqueous redox-flow batteries that incorporate NaSICON membranes, illustrating the importance of SCC in cell design. This work provides quantitative insights into SCC as a failure mode in NaSICON, enhancing our understanding of the chemo-mechanical behavior of ceramic electrolytes in contact with aqueous solutions.
Sabrina Weber et al 2025 J. Electrochem. Soc. 172 044506
Solid oxide fuel cells (SOFCs) are becoming increasingly important due to their high electrical efficiency, the flexible choice of fuels and relatively low emissions of pollutants. However, the increasingly growing demands for electrochemical devices require further performance improvements as for example by reducing degradation effects. Since it is well known that the 3D electrode morphology, which is significantly influenced by the underlying manufacturing process, has a profound impact on the resulting performance, a deeper understanding for the structural changes caused by modifications of the manufacturing process or degradation phenomena is desirable. In the present paper, we investigate the influence of the annealing time and the operating temperature on the 3D morphology of SOFC anodes using 3D image data obtained by focused-ion beam scanning electron microscopy, which is segmented into gadolinium-doped ceria, nickel and pore space. In addition, structural differences caused by manufacturing the anode via infiltration or powder technology, respectively, are analyzed quantitatively by means of various geometrical descriptors such as specific surface area, mean geodesic tortuosity, and constrictivity. The computation of these descriptors from 3D image data is carried out both globally as well as locally to quantify the heterogeneity of the anode structure.
Taylor R. Garrick et al 2025 J. Electrochem. Soc.
In this work we demonstrate the applicability of a versatile separator-reference mounted electrode in a three-electrode setup to accurately capture the primary current pathway in the battery cell during operation, calibrate a porous electrode model to the individual anode and cathode potential signals, and assign the various resistances to electrochemical phenomena in the battery cell. This calibrated electrochemical model is validated using continuous rate discharges associated with highway driving scenarios in an electric vehicle, and in turn utilized to predict the local anode potential and proximity to lithium plating onset. Finally, we demonstrate the strategy associated with utilization of the model to estimate constant anode potential charging during fast charge scenarios at various rates and starting conditions as a future look to fast charge calibration development and controls.
Hamid Reza Abbasi et al 2025 J. Electrochem. Soc. 172 044505
The design of the microporous structure of solid oxide fuel cell (SOFC) anodes can significantly affect the overall cell efficiency. A novel pore-scale computational framework has been developed using Plurigaussian methods to create micorporous anode topologies at will. A multi-dimensional model describing the electrochemical phenomena occurring within the corresponding discretised domain of the active layer of the porous anode cermet has been constructed and solved using the finite volume method. Three different anode configurations have been computationally synthesized and analyzed with respect to their topological properties and electrochemical performance: A conventional Ni/YSZ configuration, and two novel designs, a fibrous and a lattice microstructure are synthesized and tested. Utilization of synthetically generated microstructures enables the direct study of microporous and image acquisition attributes. Our numerical investigations demonstrate that lattice and fibrous structures produce increased current densities by 4.8 and 1.4 times, respectively, compared to conventional configurations. In addition, gradient microstructures also have the capacity to enhance electrochemical performance when subjected to careful fabrication methodologies.
Carlos Mejia and Devin Rappleye 2025 J. Electrochem. Soc. 172 043502
Molten salt electrochemistry has not been widely studied compared to aqueous and ionic liquids, and an experimental methodology to perform those experiments is crucial to the molten salt community. One of the main challenges in performing these experiments is the inability to visualize the position of the electrodes or the condition of the molten bath. In some cases, the placement of electrodes and the temperature of the molten bath can raise problems when the electrodes are not in contact with the molten salt or when the electrodes are in direct contact with each other. This methodology study investigates scenarios where a molten salt experiment can fail. This methodology study also provides new approaches to enhance and provide more accurate results when performing electrochemical analysis in molten salts to obtain prominent properties, such as the diffusion coefficient. Even though this paper does not involve determining those properties, it provides general guidelines and suggestions to improve the quality of electrochemical data and troubleshoot experimental setups.
Highlights
A clear methodology improves molten salt electrochemical experiment reliability.
Challenges with electrode placement and molten salt temperatures are addressed.
iR compensation and surface roughness effects on accuracy are carefully examined.
Insights into quasi-reference electrodes reveal their limits in molten salt systems.
Practical advice enhances the quality and success of molten salt experiments.
Katherine Betts et al 2025 J. Electrochem. Soc.
An in situ double probe beam deflection (PBD) technique has been developed using two laser beams to map the concentration profile of the diffusion layer in an electrochemical cell. A microscale moving upper probe and a fixed position secondary beam offer real-time concentration gradients to be profiled throughout the depth of the diffusion layer. The double PBD technique was used to plot concentration profiles for 0.1 mol/kg CuSO4 and ZnSO4 within a range of applied currents, showing increased magnitudes of gradients for higher currents. Both single and double beam PBD were explored, demonstrating the distance and time dependence of the developing concentration gradient. While CuSO4 showed a systematic trend of increased response delay and decreased deflection with increased distance from the electrode, ZnSO4 experienced some additional phenomena affecting the refractive index within the diffusion layer. The in situ double probe beam deflection was shown to be highly sensitive and offers future work in quantifying charge migration within this important region of the electrochemical cell.
Darby Hickson and Nitash P Balsara 2025 J. Electrochem. Soc.
The passage of current through a battery results in the development of concentration gradients in the electrolytic phase. For a fully characterized binary electrolyte, where the conductivity, salt diffusion coefficient, cation transference number, and the thermodynamic factor are known, concentration and potential gradients in the electrolytic phase can be modeled using Newman’s concentrated solution theory. We report two methods for measuring the transference number: the standard method based on electrochemical measurements (t_(+,echem)^0) and electrophoretic NMR (t_(+,eNMR)^0). The electrochemical approach requires combining measurements from multiple experiments; the equations used to determine the cation transference number and the thermodynamic factor are coupled, nonlinear algebraic equations. In the electrophoretic-NMR-based approach, however, the equations used to determine the cation transference number and the thermodynamic factor are decoupled. We find for a liquid electrolyte comprised of a lithium salt dissolved in tetraglyme, the values of the transference numbers obtained by these two methods are distinct. For example, at 30C, t_(+,echem)^0 = -1.02±1.11 and t_(+,eNMR)^0 = 0.25±0.04. The corresponding thermodynamic factors are also different. While the magnitude of the predicted concentration gradients based on the two sets of parameters are different, the predicted current-voltage relationships are similar.
Nobuyuki Serizawa et al 2025 J. Electrochem. Soc.
The physicochemical and electrochemical properties of an ionic liquid consisting of the dichlorocuprate anion, [CuCl2]–, were investigated. The equimolar mixture of CuCl and 1-butyl-1-methylpyrrolidinium chloride (BMPCl) was found to yield an ionic liquid at 298 K composed predominantly of BMP+ and [CuCl2]–. Fine deposits of metallic Cu were obtained on a glassy carbon electrode by potentiostatic and galvanostatic reduction of [CuCl2]– in CuCl-BMPCl (50.0-50.0 mol%). The overpotential for Cu nucleation was larger on a glassy carbon electrode than on a Pt electrode. The electrochemical deposition and dissolution of Cu were analyzed using an electrochemical quartz crystal microbalance. The local viscosity and density of the electrolyte near the electrode/electrolyte interface increased during both deposition and dissolution of Cu, probably reflecting the shift in the electrolyte composition to the local basic and acidic conditions, respectively.