As service lifetimes of electric vehicle (EV) and grid storage batteries continually improve, it has become increasingly important to understand how cells perform after extensive cycling. The multifaceted nature of degradation in Li-ion cells can lead to complex behavior that may be difficult for battery management systems or operators to model. Accurate characterization of heavily cycled cells is critical for developing accurate models, especially for cycle-intensive applications like second-life grid storage or vehicle-to-grid charging. In this study, we use operando synchrotron x-ray diffraction (SR-XRD) to characterize a commercially manufactured polycrystalline NMC622 pouch cell that was cycled for more than 2.5 years. Using spatially resolved synchrotron XRD, the complex kinetics and spatially heterogeneous behavior of such cells are mapped and characterized under both near-equilibrium and non-equilibrium conditions. The resulting data is complex and multifaceted, requiring a different approach to analysis and modelling than what has been used in the literature. To show how material selection can impact the extent of degradation, we compare the results from polycrystalline NMC622 cells to an extensively cycled single-crystal NMC532 cell with over 20,000 cycles—equivalent to a total EV traveled distance of approximately 8 million km (5 million miles) over six years.

The Electrochemical Society (ECS) was founded in 1902 to advance the theory and practice at the forefront of electrochemical and solid state science and technology, and allied subjects.
ISSN: 1945-7111
JES is the flagship journal of The Electrochemical Society. Published continuously from 1902 to the present, JES remains one of the most highly-cited journals in electrochemistry and solid-state science and technology.
Toby Bond et al 2024 J. Electrochem. Soc. 171 110514
Manuel Ank et al 2023 J. Electrochem. Soc. 170 120536
Battery research depends upon up-to-date information on the cell characteristics found in current electric vehicles, which is exacerbated by the deployment of novel formats and architectures. This necessitates open access to cell characterization data. Therefore, this study examines the architecture and performance of first-generation Tesla 4680 cells in detail, both by electrical characterization and thermal investigations at cell-level and by disassembling one cell down to the material level including a three-electrode analysis. The cell teardown reveals the complex cell architecture with electrode disks of hexagonal symmetry as well as an electrode winding consisting of a double-sided and homogeneously coated cathode and anode, two separators and no mandrel. A solvent-free anode fabrication and coating process can be derived. Energy-dispersive X-ray spectroscopy as well as differential voltage, incremental capacity and three-electrode analysis confirm a NMC811 cathode and a pure graphite anode without silicon. On cell-level, energy densities of 622.4 Wh/L and 232.5 Wh/kg were determined while characteristic state-of-charge dependencies regarding resistance and impedance behavior are revealed using hybrid pulse power characterization and electrochemical impedance spectroscopy. A comparatively high surface temperature of ∼70 °C is observed when charging at 2C without active cooling. All measurement data of this characterization study are provided as open source.
Eniko S. Zsoldos et al 2024 J. Electrochem. Soc. 171 080527
Lithium iron phosphate (LFP) battery cells are ubiquitous in electric vehicles and stationary energy storage because they are cheap and have a long lifetime. This work compares LFP/graphite pouch cells undergoing charge-discharge cycles over five state of charge (SOC) windows (0%–25%, 0%–60%, 0%–80%, 0%–100%, and 75%–100%). Cycling LFP cells across a lower average SOC results in less capacity fade than cycling across a higher average SOC, regardless of depth of discharge. The primary capacity fade mechanism is lithium inventory loss due to: lithiated graphite reactivity with electrolyte, which increases incrementally with SOC, and lithium alkoxide species causing iron dissolution and deposition on the negative electrode at high SOC which further accelerates lithium inventory loss. Our results show that even low voltage LFP systems (3.65 V) have a tradeoff between average SOC and lifetime. Operating LFP cells at lower average SOC can extend their lifetime substantially in both EV and grid storage applications.
Peter Keil et al 2016 J. Electrochem. Soc. 163 A1872
In this study, the calendar aging of lithium-ion batteries is investigated at different temperatures for 16 states of charge (SoCs) from 0 to 100%. Three types of 18650 lithium-ion cells, containing different cathode materials, have been examined. Our study demonstrates that calendar aging does not increase steadily with the SoC. Instead, plateau regions, covering SoC intervals of more than 20%–30% of the cell capacity, are observed wherein the capacity fade is similar. Differential voltage analyses confirm that the capacity fade is mainly caused by a shift in the electrode balancing. Furthermore, our study reveals the high impact of the graphite electrode on calendar aging. Lower anode potentials, which aggravate electrolyte reduction and thus promote solid electrolyte interphase growth, have been identified as the main driver of capacity fade during storage. In the high SoC regime where the graphite anode is lithiated more than 50%, the low anode potential accelerates the loss of cyclable lithium, which in turn distorts the electrode balancing. Aging mechanisms induced by high cell potential, such as electrolyte oxidation or transition-metal dissolution, seem to play only a minor role. To maximize battery life, high storage SoCs corresponding to low anode potential should be avoided.
George E. Blomgren 2017 J. Electrochem. Soc. 164 A5019
This year, the battery industry celebrates the 25th anniversary of the introduction of the lithium ion rechargeable battery by Sony Corporation. The discovery of the system dates back to earlier work by Asahi Kasei in Japan, which used a combination of lower temperature carbons for the negative electrode to prevent solvent degradation and lithium cobalt dioxide modified somewhat from Goodenough's earlier work. The development by Sony was carried out within a few years by bringing together technology in film coating from their magnetic tape division and electrochemical technology from their battery division. The past 25 years has shown rapid growth in the sales and in the benefits of lithium ion in comparison to all the earlier rechargeable battery systems. Recent work on new materials shows that there is a good likelihood that the lithium ion battery will continue to improve in cost, energy, safety and power capability and will be a formidable competitor for some years to come.
Yuliya Preger et al 2020 J. Electrochem. Soc. 167 120532
Energy storage systems with Li-ion batteries are increasingly deployed to maintain a robust and resilient grid and facilitate the integration of renewable energy resources. However, appropriate selection of cells for different applications is difficult due to limited public data comparing the most commonly used off-the-shelf Li-ion chemistries under the same operating conditions. This article details a multi-year cycling study of commercial LiFePO4 (LFP), LiNixCoyAl1−x−yO2 (NCA), and LiNixMnyCo1−x−yO2 (NMC) cells, varying the discharge rate, depth of discharge (DOD), and environment temperature. The capacity and discharge energy retention, as well as the round-trip efficiency, were compared. Even when operated within manufacturer specifications, the range of cycling conditions had a profound effect on cell degradation, with time to reach 80% capacity varying by thousands of hours and cycle counts among cells of each chemistry. The degradation of cells in this study was compared to that of similar cells in previous studies to identify universal trends and to provide a standard deviation for performance. All cycling files have been made publicly available at batteryarchive.org, a recently developed repository for visualization and comparison of battery data, to facilitate future experimental and modeling efforts.
Chang-Hui Chen et al 2020 J. Electrochem. Soc. 167 080534
Presented here, is an extensive 35 parameter experimental data set of a cylindrical 21700 commercial cell (LGM50), for an electrochemical pseudo-two-dimensional (P2D) model. The experimental methodologies for tear-down and subsequent chemical, physical, electrochemical kinetics and thermodynamic analysis, and their accuracy and validity are discussed. Chemical analysis of the LGM50 cell shows that it is comprised of a NMC 811 positive electrode and bi-component Graphite-SiOx negative electrode. The thermodynamic open circuit voltages (OCV) and lithium stoichiometry in the electrode are obtained using galvanostatic intermittent titration technique (GITT) in half cell and three-electrode full cell configurations. The activation energy and exchange current coefficient through electrochemical impedance spectroscopy (EIS) measurements. Apparent diffusion coefficients are estimated using the Sand equation on the voltage transient during the current pulse; an expansion factor was applied to the bi-component negative electrode data to reflect the average change in effective surface area during lithiation. The 35 parameters are applied within a P2D model to show the fit to experimental validation LGM50 cell discharge and relaxation voltage profiles at room temperature. The accuracy and validity of the processes and the techniques in the determination of these parameters are discussed, including opportunities for further modelling and data analysis improvements.
D. Noel Buckley and Johna Leddy 2024 J. Electrochem. Soc. 171 116503
We revisit the classical derivation of the Butler-Volmer equation to include the effect of the electrode metal. If the metal is replaced by one with a different work function, keeping other conditions in the electrode constant, the chemical potential of electrons  and the Galvani potential
and the Galvani potential  change in a complementary manner. Changes in
change in a complementary manner. Changes in  and
and  each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of
each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of  Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both
Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both  and
and  The exchange current density
The exchange current density  appears as an exponential function of
appears as an exponential function of  For the work function
For the work function  of the metal, the approximation
of the metal, the approximation  yields a linear relationship between
yields a linear relationship between  and
and  The linear increase in
The linear increase in  with
with  has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
figure placeholder
Matthew D. L. Garayt et al 2024 J. Electrochem. Soc. 171 120521
Sodium-ion batteries (NIBs) are of growing interest due to their expected lower cost than many lithium-ion batteries (LIBs). However, most NIBs suffer from lower volumetric energy density than LIBs. Lead (Pb) can replace hard carbon in the NIB negative electrode to substantially increase its volumetric energy density and has been shown to have no capacity fade over hundreds of cycles in half cells. Pb also experiences 387% volume expansion upon full sodiation, which presumably leads to significant changes in the electrode morphology. In this work, the morphology of Pb and Pb-hard carbon blended electrodes is tracked using scanning electron microscopy. As well, each Na-Pb phase is examined to analyze their physical properties. These analyses show that the Pb particles restructure into ∼1 μm particles, even after just a single cycle, and surprisingly do not pulverize the hard carbon in a blended electrode. Importantly, single-walled carbon nanotubes appear to be necessary to maintain active material electrical connection during the restructuring.
Xia Cao et al 2021 J. Electrochem. Soc. 168 010522
The conventional LiPF6/carbonate-based electrolytes have been widely used in graphite (Gr)-based lithium (Li) ion batteries (LIBs) for more than 30 years because a stable solid electrolyte interphase (SEI) layer forms on the graphite surface and enables its long-term cycling stability. However, few of these electrolytes are stable under the more stringent conditions needed with a Li metal anode (LMA) and other anodes, such as silicon (Si), which exhibit large volume changes during charge/discharge processes. Many different approaches have been developed lately to stabilize Li metal batteries (LMBs) and Si-based LIBs. From this aspect, localized high-concentration electrolytes (LHCEs) have unique advantages: not only are they stable in a wide electrochemical window, they can also form stable SEI layers on LMA and Si anode surfaces to enable their long-term cycling stability. The ultrathin SEI layer formed on a Gr anode can also improve the safety and high-rate operation of conventional LIBs. In this paper, we give a brief summary of our recent work on LHCEs, including their design principle and applications in both LMBs and LIBs. A perspective on the future development of LHCEs is also discussed.
Anand Kumar Agrawal et al 2025 J. Electrochem. Soc. 172 013501
The electrochemical response, and hence the performance, of lithium-ion batteries (LIBs) is heavily influenced by the microstructure of their electrode material, particularly grain size. Thus, a model that correlates electrode microstructure with electrochemical response can aid in optimizing these characteristics for better battery performance. However, no existing studies address this modeling problem. This study fills this gap by using data-driven probabilistic modeling to map the electrochemical response (voltammograms) to electrode grain size. The methodology is demonstrated on six datasets containing voltammograms from in-silico cyclic voltammetry experiments on LiMn2O4 electrode particles. Feature vectors created using seven features of the voltammogram were combined with the corresponding known grain size values to construct data-driven probabilistic models via Gaussian process regression (GPR) with five different kernels for each dataset. The hyperparameters of the kernels used in these GPR models were optimized using grid-search. Performance evaluation through bias-variance analysis demonstrated strong predictive accuracy of the developed models. Additionally, the comparisons across datasets and kernels showed the Gaussian and Rational Quadratic kernels' effectiveness in modeling various relationships. The obtained results substantiate the efficacy of the proposed approach, revealing significant insights and encouraging further exploration.
Vishal Chaudhary 2025 J. Electrochem. Soc. 172 017501
Semiconductors with nanoscale dimensions are indispensable vectors for devising modern-age electronics-enabled technologies. Meeting the rising technological demand of the globally expanding population, while limiting the cost to the ecosystem, necessitates the sustainable development of green semiconductors at the nanoscale. This perspective highlights the state-of-the-art green nano-semiconductors, including metal oxides, organic materials, and hybrid nanosystems, with three key challenges: scalability, stability, and susceptibility. It also discusses alternate solutions integrating modern technologies like artificial intelligence to establish these green nano-semiconductors as a sustainable frontier to revolutionize multidimensional applications such as sensors, medicines, electronics, energy systems, and environmental remediation while minimizing ecological footprints.
Payman Sharifi Abdar et al 2025 J. Electrochem. Soc. 172 011501
With the increase in production of sour oil and gas fields, mitigation of production-related failures due to H2S corrosion of mild steel is a key challenge. In H2S environments, most failures occur due to localized corrosion originating from the galvanic coupling between mild steel and conductive iron sulfide corrosion products. However, the mechanism of the galvanic coupling between mild steel and iron sulfides and the effect of influential parameters, have not been studied yet. Here, we provide a systematic experimental investigation on the galvanic corrosion between mild steel and iron sulfides by examining the effect of the critical factors: iron sulfide type, cathode to anode surface area ratio, and salt concentration. Specifically, we focus on pyrite and pyrrhotite as the main corrosion products found in localized corrosion of mild steel in H2S environments. Our results show that the cathodic current obtained on pyrrhotite was an order of magnitude higher than that obtained on pyrite, leading to a higher galvanic current for coupled mild steel-pyrrhotite compared to coupled mild steel-pyrite. Moreover, our study reveals that the increase of cathode to anode surface area ratio and, to some extent, the increase of salt concentration, enhance the galvanic current for the coupled materials.
Qipeng Zhang et al 2025 J. Electrochem. Soc. 172 010501
Sodium metal batteries (SMBs) are cost-effective and environmentally sustainable alternative to lithium batteries. However, at present, limitations such as poor compatibility, low coulombic efficiency (CE), and high electrolyte cost hinder their widespread application. Herein, we propose a non-flammable, low-concentration electrolyte composed of 0.3 M NaPF6 in propylene carbonate (PC), fluoroethylene carbonate (FEC), and 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether (TTE). This low-concentration electrolyte not only reduces cost but also delivers rapid ion diffusion and superior wetting properties. While the Na||FePO4 system with this electrolyte demonstrates slightly reduced performance at room temperature compared to standard-concentration formulations (S-PFT), it excels at both high (55 °C) and low (−20 °C) temperatures, showcasing its balanced performance. At 0.5 C (charge)/1 C (discharge), capacity retention reaches 92.8% at room temperature and 98.5% at elevated temperature, with CE values surpassing 99% and 99.63%, respectively, and significant performance sustained at −20 °C at 0.2 C. This electrolyte development thus offers a well-rounded, economically viable path to high-performance SMBs for diverse environmental applications.
Weiwei Xu et al 2024 J. Electrochem. Soc. 171 120545
The high cost and uneven distribution of lithium resources have prompted searches for alternatives to lithium-ion batteries. Among various alternatives, the sodium layered oxide cathode materials, have shown significant research potential due to their low cost. Layered oxide materials can be categorized into sodium-rich O3 types and sodium-deficient P2 types, which have different structural features. O3 type materials offer high specific capacities but suffer from complex pathways for Na+ de-intercalation, slow Na+ diffusion, and poor air stability. P2 type materials are limited in full cell applications due to their lower practical specific capacities. Therefore, researchers conceived the idea of combining the advantages of both to construct P2/O3 composite structure cathode materials (CSMs), utilizing the synergistic effects of the CSMs to overcome the limitations of single structure material, and successfully synthesized CSMs with appropriate specific capacities. These materials effectively suppress unfavorable phase transitions and enhance Na+ diffusion coefficient, thereby improving electrochemical performance. This paper reviews the recent advancements in CSMs for sodium-ion batteries, highlighting synthesis strategies that incorporate "cationic potential" theory, element substitution, sodium content adjustment, and control of calcination processes to synthesize diverse CSMs.
Weiwei Xu et al 2024 J. Electrochem. Soc. 171 120545
The high cost and uneven distribution of lithium resources have prompted searches for alternatives to lithium-ion batteries. Among various alternatives, the sodium layered oxide cathode materials, have shown significant research potential due to their low cost. Layered oxide materials can be categorized into sodium-rich O3 types and sodium-deficient P2 types, which have different structural features. O3 type materials offer high specific capacities but suffer from complex pathways for Na+ de-intercalation, slow Na+ diffusion, and poor air stability. P2 type materials are limited in full cell applications due to their lower practical specific capacities. Therefore, researchers conceived the idea of combining the advantages of both to construct P2/O3 composite structure cathode materials (CSMs), utilizing the synergistic effects of the CSMs to overcome the limitations of single structure material, and successfully synthesized CSMs with appropriate specific capacities. These materials effectively suppress unfavorable phase transitions and enhance Na+ diffusion coefficient, thereby improving electrochemical performance. This paper reviews the recent advancements in CSMs for sodium-ion batteries, highlighting synthesis strategies that incorporate "cationic potential" theory, element substitution, sodium content adjustment, and control of calcination processes to synthesize diverse CSMs.
Chenchen Liu et al 2024 J. Electrochem. Soc. 171 120527
Safety issues have hindered the rapid development of lithium-ion batteries for use in energy storage and vehicles, especially the frequent battery thermal runaway (TR) accidents. The TR of lithium-ion batteries can result in fire and explosion. Understanding the thermal runaway mechanisms and triggers is key to optimizing early warning strategies. Here, we provide a comprehensive review from three aspects: trigger, mechanism, and early warning strategy. By analyzing typical incidents, both external abuses and internal defects are identified as key triggers of TR. The energy release mechanisms during TR are explored through multi-physics coupling models, leading to the development of a TR safety-phase diagram. The primary exothermic reactions and heat generation pathways are summarized, with a focus on the contribution of side reactions in various material systems. Furthermore, early warning strategies are reviewed, including single-signal and multi-physics characteristic signal analysis, highlighting the technical challenges for future TR safety predictions. This review enhances the understanding of TR mechanisms and is crucial for advancing battery safety.
Murali Rangarajan et al 2024 J. Electrochem. Soc. 171 124502
Carbon dioxide (CO2) capture and storage are now an essential reality that we are required to adapt to address global climate change concerns. Adapting carbon neutrality or carbon negative processes in mainstream energy generation, manufacturing, and transportation is possible using current technologies, albeit with some limitations. Carbon neutral technologies (CNTs) can be seamlessly integrated with existing systems as well as green technologies to ensure that carbon capture gets a boost. On-land and undersea storage are realistic possibilities since there is immense potential to lock atmospheric CO2 using existing technologies. Thermocatalytic, electrochemical, photo(electro)catalytic, and biological—based approaches do offer promising options, but require optimization of different parameters to ensure commercial viability, scalability, and safety. The role of electrochemical process specifically is examined. New directions for further research in the area of electrochemical—driven applications are identified and opportunities in three areas, viz., electrocatalysts design, pilot scale integrated systems, and simultaneous CO2 capture and conversion, are discussed in detail. The global implementation of any CNTs requires dramatic policy shift, unequivocal support from the world governments, public acceptance, backing from industries, and unwavering financial backing from stakeholders to ensure that there is a real chance to address climate change issues.
Bhuvsmita Bhargava et al 2024 J. Electrochem. Soc. 171 110525
The use of differential scanning calorimetry (DSC) to measure the thermal behavior of individual components and electrolyte/electrode combinations is common. However, here we focus on DSC tests on an anode, cathode, and electrolyte (ACE) component combination over a temperature range that includes many of the phase transitions and key reactions (i.e., to 500 °C) that contribute to thermal runaway. This method can help quantify the complex reaction network in a full cell, thereby informing potential safety issues. Here, we used DSC heat flow data from a solid-state Li0.43CoO2+C+PVDF | LLZO | Li metal ACE sample and its components to quantify key factors affecting results. We focused on three areas: (1) ACE sample preparation and assembly in DSC pans, (2) DSC measurement parameters, and (3) heat flow analysis. Key points include the choice of component ratios (e.g., commercially relevant N:P capacity ratio), the importance of conductive carbon and binder, type of pan used, DSC ramp rate, and integration method used when dealing with broad and overlapping exothermic peaks. This work deepens the scientific basis and best practices for obtaining heat flow data from ACE samples for early-stage evaluation of solid-state and beyond battery safety.
Shweta Meena 2024 J. Electrochem. Soc. 171 117528
Layered two-dimensional materials have gained tremendous attention in the area of bio sensing. Among two-dimensional materials (2D), MXenes have been recognized as versatile material for advanced biosensing applications. Recently, MXenes have gained huge popularity due to their good biocompatibility, high metallic conductivity, exceptional hydrophilicity, ease of surface functionalization, high surface area, better redox ability, and high heterogeneous transfer rate of electron. Antitoxicity and anti-fouling properties, hydrophilic behaviour, and biocompatibility have unfolded new avenues for MXenes in performing in vivo and in vitro analysis. This review comprehensively assesses the basic concept and distinctive properties of MXenes along with MXene synthesis and strategies in the growth of different wearable, immunosensors, optical and electrochemical biosensors. In addition, challenges in the usage of MXenes for biosensors are compiled with future scope. This review is considered to elucidate the growth of MXenes in biosensing and is believed to open possibilities in translational applications for MXene bio-assays and development of advanced MXene based biosensors having higher selectivity and sensitivity.
D. Noel Buckley and Johna Leddy 2024 J. Electrochem. Soc. 171 116503
We revisit the classical derivation of the Butler-Volmer equation to include the effect of the electrode metal. If the metal is replaced by one with a different work function, keeping other conditions in the electrode constant, the chemical potential of electrons  and the Galvani potential
and the Galvani potential  change in a complementary manner. Changes in
change in a complementary manner. Changes in  and
and  each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of
each impact the free energies of activation of the forward and backward electron transfer reactions, so we modify the classical expressions which relate them to applied voltage E by including also the effect of  Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both
Inserting these expressions in an Eyring-Polyani or Arrhenius type equation in the traditional way, we obtain a modified Butler-Volmer equation which expresses current density as a function of both  and
and  The exchange current density
The exchange current density  appears as an exponential function of
appears as an exponential function of  For the work function
For the work function  of the metal, the approximation
of the metal, the approximation  yields a linear relationship between
yields a linear relationship between  and
and  The linear increase in
The linear increase in  with
with  has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
has long been reported. We show two experimental examples: the aqueous Fe2+/Fe3+ couple with positive slope and the hydrogen evolution reaction (HER) with parallel lines for the d and sp metals, both with positive slopes.
figure placeholder
Philip Minnmann et al 2024 J. Electrochem. Soc. 171 060514
The kinetics of composite cathodes for solid-state batteries (SSBs) relies heavily on their microstructure. Spatial distribution of the different phases, porosity, interface areas, and tortuosity factors are important descriptors that need accurate quantification for models to predict the electrochemistry and mechanics of SSBs. In this study, high-resolution focused ion beam-scanning electron microscopy tomography was used to investigate the microstructure of cathodes composed of a nickel-rich cathode active material (NCM) and a thiophosphate-based inorganic solid electrolyte (ISE). The influence of the ISE particle size on the microstructure of the cathode was visualized by 3D reconstruction and charge transport simulation. By comparison of experimentally determined and simulated conductivities of composite cathodes with different ISE particle sizes, the electrode charge transport kinetics is evaluated. Porosity is shown to have a major influence on the cell kinetics and the evaluation of the active mass of electrochemically active particles reveals a higher fraction of connected NCM particles in electrode composites utilizing smaller ISE particles. The results highlight the importance of homogeneous and optimized microstructures for high performance SSBs, securing fast ion and electron transport.
S. Yanev et al 2024 J. Electrochem. Soc. 171 020512
Li-In electrodes are widely applied as counter electrodes in fundamental research on Li-metal all-solid-state batteries. It is commonly assumed that the Li-In anode is not rate limiting, i.e. the measurement results are expected to be representative of the investigated electrode of interest. However, this assumption is rarely verified, and some counterexamples were recently demonstrated in literature. Herein, we fabricate Li-In anodes in three different ways and systematically evaluate the electrochemical properties in two- and three-electrode half-cells. The most common method of pressing Li and In metal sheets together during cell assembly resulted in poor homogeneity and low rate performance, which may result in data misinterpretation when applied for investigations on cathodic phenomena. The formation of a Li-poor region on the separator side of the anode is identified as a major kinetic bottleneck. An alternative fabrication of a Li-In powder anode resulted in no kinetic benefits. In contrast, preparing a composite from Li-In powder and sulfide electrolyte powder alleviated the kinetic limitation, resulted in superior rate performance, and minimized the impedance. The results emphasize the need to fabricate optimized Li-In anodes to ensure suitability as a counter electrode in solid-state cells.

Highlights
The fabrication of Li-In anodes needs to be optimized to ensure suitability as a counter electrode in sulfide all-solid-state batteries.
The Li-In counter electrode may often be the limiting factor of sulfide all-solid-state halfcells.
Pressing Li and In foil together results in a kinetically limited anode.
Composites from Li-In and sulfide electrolyte result in stable reference potential, superior rate performance and low impedance of the counter electrode.
Ramver Singh et al 2024 J. Electrochem. Soc. 171 013501
Electrical discharge micromachining (EDM) poses challenges to the fatigue-life performance of machined surfaces due to thermal damage, including recast layers, heat-affected zones, residual stress, micro-cracks, and pores. Existing literature proposes various ex situ post-processing techniques to mitigate these effects, albeit requiring separate facilities, leading to increased time and costs. This research involves an in situ sequential electrochemical post-processing (ECPP) technique to enhance the quality of EDMed micro-holes on titanium. The study develops an understanding of the evolution of overcutting during ECPP, conducting unique experiments that involve adjusting the initial radial interelectrode gap (utilizing in situ wire-electrical discharge grinding) and applied voltage. Additionally, an experimentally validated transient finite element method (FEM) model is developed, incorporating the passive film formation phenomenon for improved accuracy. Compared to EDM alone, the sequential EDM-ECPP approach produced micro-holes with superior surface integrity and form accuracy, completely eliminating thermal damage. Notably, surface roughness (Sa) was reduced by 80% after the ECPP. Increasing the voltage from 8 to 16 V or decreasing the gap from 60 to 20 μm rendered a larger overcut. This research's novelty lies in using a two-phase dielectric (water-air), effectively addressing dielectric and electrolyte cross-contamination issues, rendering it suitable for commercial applications.

Highlights
Better micro-hole quality through in situ sequential eco-friendly near-dry EDM & ECM
Successfully resolved dielectric-electrolyte cross-contamination in sequential processes
Unique experiments that adjust the initial radial IEG using in situ wire-EDG
Developed and validated a transient FEM model, incorporating passivation aspect
Achieved recast layer-free holes with Sa values approximately 80% lower than EDM holes
D. A. Sudarikov et al 2023 J. Electrochem. Soc. 170 086505
Vanadium dioxide is widely known for its metal-insulator transition (MIT), in which drastic changes in resistivity and IR-transparency occur. This makes VO2 thin films promising materials for high-frequency optoelectronic devices. To get the most MIT sharpness, thin films should not contain impurities of hyper-oxygen or hypo-oxygen phases arising during VO2 synthesis. To ascertain the conditions of single-phase VO2 existence, the equilibrium boundaries of VO2 with neighboring phases were determined using the electromotive force method (EMF) with a solid electrolyte ZrO2(Y2O3). Our data for the high-oxygen boundary of VO2 existence in equilibrium with the V6O13 phase agree with the only data known in the literature. We established that VO2 is, in equilibrium with the V9O17 phase at the low-oxygen boundary, which forms V8O15 under further reduction. The temperature of the peritectoid decomposition of V9O17 is established, and the corresponding corrections to the phase diagram of the vanadium-oxygen system are introduced. The Gibbs energies for V9O17, V8O15, and V6O13 formation reactions are calculated. It is also shown that the IR reflectance of VO2 films brought to equilibrium at the high-oxygen boundary is much greater than that of films equilibrated at the low-oxygen boundary.
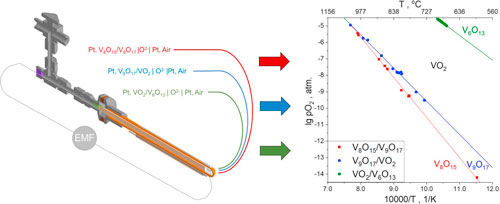
Highlights
Equilibrium boundaries of VO2 phase stability were studied by the EMF method.
Low-oxygen boundary revealed more complex equilibriums than previously assumed.
Thermodynamic data about equilibriums of V8O15, V9O17, VO2, V6O13 were obtained.
New information was added to the phase diagram of the vanadium-oxygen system.
Influence of nonstoicometry on MIT was shown by IR experiments with thin VO2 films.
Tao et al
This study introduces the development of a portable electrochemical sensor for the detection of Pb(II) and Cd(II) ions based on a screen-printed graphene electrode (SPGE) enhanced with alkalized MXene and gold nanoparticles (AuNPs). Experimental results reveal that the Au@Alk-MXene/SPGE electrode exhibits excellent electrical conductivity, enlarged active surface area, and efficient electron transfer capability, which enhances the electrochemical detection of Pb(II) and Cd(II) ions. Under optimal experimental conditions, the sensor demonstrates good electrochemical response and distinct stripping peaks for both ions. The linear detection range of the sensor is 5-130 μg L⁻¹ for both Pb(II) and Cd(II), with detection limits of 0.198 μg L⁻¹ for Pb(II) and 0.106 μg L⁻¹ for Cd(II). Furthermore, the sensor shows excellent repeatability with relative standard deviations (RSDs) of 1.16% (n = 10) for Pb(II) and 1.25% (n = 10) for Cd(II), as well as remarkable reproducibility with RSD values of 1.56% for Pb(II) and 1.57% for Cd(II). The sensor was successfully applied to analyze tap water and lake water samples, demonstrating satisfactory recovery rates. This research shows the use of MXenes and AuNPs to modify SPGE electrodes, offering a rapid and portable electrochemical sensor for detecting Pb(II) and Cd(II) ions in water.
Chen et al
Human epididymal protein (HE4) is the most sensitive and specific tumor marker of ovarian cancer (OC). Therefore, it is necessary to achieve sensitive and selective determination of HE4 in humans. Due to the high sensitivity and fast response of the electrochemical method, the main research method for HE4 detection is still electrochemical method. Here we review recent research progress of electrochemical (EC), electrochemiluminescence (ECL), optical (OP), and photoelectrochemical (PEC) immunobiosensors for OC tumor marker HE4 detection. Because of the special physical and chemical properties of carbon nanomaterials, precious metal nanomaterials and quantum dots, they are widely used in immunosensors design. Exploring new nanocomposites to improve electrode performance is a research direction to improve the sensitivity and selectivity of sensors in the future. The advantages and limitations of the above four sensors and the future development direction of HE4 immunosensors are reviewed and discussed.
Demnitz et al
Alkaline water electrolysis using catalyst coated diaphragms (Zirfon UTP 500 and UTP 220) was conducted at current densities from 2 up to 3500 mA/cm2 at varying temperatures (20 – 75°C) in 30 wt.% KOH. The coatings were conducted using two different approaches, which were compared with each other: spray coating and stencil coating. Using platinum group free catalysts, which are either available commercially or easy to synthesize (Raney Ni; FeNi LDH), we reached 3.5 A/cm2 at less than 2.3 or 2.5 V, for Zirfon UTP 220 and 500, respectively. The improvements compared to conventional Ni felt were linked to a reduction in the kinetic overpotential due to better catalytic properties and an increase in active surface area. The internal resistance corrected potential at 1 A/cm2 was as low as 1.75 V (at 75°C), showing that high current operation for industrial alkaline water electrolysers is possible, when ohmic resistances are adequately addressed. The catalyst coated diaphragms were stable under room temperature for at least 60 h, however, showed degradation at 75°C over the course of up to 240 h. The catalyst layers degraded by fracturing followed by delamination to the porous transport layer, where they showed elongated stability.
Saxena et al
Amorphous polymers have some limitations compared to their crystalline counterparts but also several advantages, such as improved transparency, ease of processing, and specific applications in industries where their unique properties are beneficial. The choice between amorphous and crystalline polymers depends on the specific requirements of the application and desired material properties. In this regard, Poly (sulfone) (PSF) and Poly (methyl methacrylate) (PMMA) offer a range of valuable properties that can be beneficial in various applications. Their unique characteristics make them stand out among other amorphous polymers and contribute to their popularity in different industries. PSF and PMMA are two distinct types of amorphous polymers that possess different chemical structures and properties, leading to varied applications. PSF is preferred when high temperature and chemical resistance are crucial, while PMMA is ideal for applications that require excellent optical clarity and transparency. Both polymers have their strengths and weaknesses, and their uses depend on specific requirements in different industries. This review aims to present a comparative analysis of the fundamental characteristics like physical, chemical, thermal, and mechanical properties of PSF and PMMA and their uses. This article also provides a valuable reference for comprehending the two polymers for progress in numerous science and technology domains
Kuwahara et al
In this work, nano-silver doped ZnO (SZO) thin films were prepared on glass substrates and through-glass vias (TGVs) using a sol-gel dip-coating method. The SZO film served as a catalytically active mixed metal oxide adhesion layer, replacing the etching, sensitization, and activation processes typically used in traditional electroless copper plating (ECP). Surface cleaning is essential for achieving high-quality coatings. In this work, an alkaline degreasing solution, followed by a citric acid chelating solution, was used to effectively enhance the wettability of the glass surface. The SZO thin film, formed on the glass substrate after dip-coating and sintering of the SZO sol, can be directly coated with a copper layer via ECP. Following subsequent electroplating, a copper film with a thickness of 11 μm and a resistivity as low as 1.84 μΩ·cm was obtained. The adhesion strength of the copper layer under vacuum annealing at 300°C in nitrogen atmosphere is 4.19 N/cm. In addition, the SZO sol-gel dip-coating process achieved complete coverage of the copper seed layer for TGVs with a high aspect ratio (10:1). This approach presented a cost-effective solution for fully solution-processed substrate-to-substrate interconnects in electronic device packaging applications.
Maximilian Demnitz et al 2025 J. Electrochem. Soc.
Alkaline water electrolysis using catalyst coated diaphragms (Zirfon UTP 500 and UTP 220) was conducted at current densities from 2 up to 3500 mA/cm2 at varying temperatures (20 – 75°C) in 30 wt.% KOH. The coatings were conducted using two different approaches, which were compared with each other: spray coating and stencil coating. Using platinum group free catalysts, which are either available commercially or easy to synthesize (Raney Ni; FeNi LDH), we reached 3.5 A/cm2 at less than 2.3 or 2.5 V, for Zirfon UTP 220 and 500, respectively. The improvements compared to conventional Ni felt were linked to a reduction in the kinetic overpotential due to better catalytic properties and an increase in active surface area. The internal resistance corrected potential at 1 A/cm2 was as low as 1.75 V (at 75°C), showing that high current operation for industrial alkaline water electrolysers is possible, when ohmic resistances are adequately addressed. The catalyst coated diaphragms were stable under room temperature for at least 60 h, however, showed degradation at 75°C over the course of up to 240 h. The catalyst layers degraded by fracturing followed by delamination to the porous transport layer, where they showed elongated stability.
Anand Kumar Agrawal et al 2025 J. Electrochem. Soc. 172 013501
The electrochemical response, and hence the performance, of lithium-ion batteries (LIBs) is heavily influenced by the microstructure of their electrode material, particularly grain size. Thus, a model that correlates electrode microstructure with electrochemical response can aid in optimizing these characteristics for better battery performance. However, no existing studies address this modeling problem. This study fills this gap by using data-driven probabilistic modeling to map the electrochemical response (voltammograms) to electrode grain size. The methodology is demonstrated on six datasets containing voltammograms from in-silico cyclic voltammetry experiments on LiMn2O4 electrode particles. Feature vectors created using seven features of the voltammogram were combined with the corresponding known grain size values to construct data-driven probabilistic models via Gaussian process regression (GPR) with five different kernels for each dataset. The hyperparameters of the kernels used in these GPR models were optimized using grid-search. Performance evaluation through bias-variance analysis demonstrated strong predictive accuracy of the developed models. Additionally, the comparisons across datasets and kernels showed the Gaussian and Rational Quadratic kernels’ effectiveness in modeling various relationships. The obtained results substantiate the efficacy of the proposed approach, revealing significant insights and encouraging further exploration.
Payman Sharifi Abdar et al 2025 J. Electrochem. Soc. 172 011501
With the increase in production of sour oil and gas fields, mitigation of production-related failures due to H2S corrosion of mild steel is a key challenge. In H2S environments, most failures occur due to localized corrosion originating from the galvanic coupling between mild steel and conductive iron sulfide corrosion products. However, the mechanism of the galvanic coupling between mild steel and iron sulfides and the effect of influential parameters, have not been studied yet. Here, we provide a systematic experimental investigation on the galvanic corrosion between mild steel and iron sulfides by examining the effect of the critical factors: iron sulfide type, cathode to anode surface area ratio, and salt concentration. Specifically, we focus on pyrite and pyrrhotite as the main corrosion products found in localized corrosion of mild steel in H2S environments. Our results show that the cathodic current obtained on pyrrhotite was an order of magnitude higher than that obtained on pyrite, leading to a higher galvanic current for coupled mild steel-pyrrhotite compared to coupled mild steel-pyrite. Moreover, our study reveals that the increase of cathode to anode surface area ratio and, to some extent, the increase of salt concentration, enhance the galvanic current for the coupled materials.
Qipeng Zhang et al 2025 J. Electrochem. Soc. 172 010501
Sodium metal batteries (SMBs) are cost-effective and environmentally sustainable alternative to lithium batteries. However, at present, limitations such as poor compatibility, low coulombic efficiency (CE), and high electrolyte cost hinder their widespread application. Herein, we propose a non-flammable, low-concentration electrolyte composed of 0.3 M NaPF6 in propylene carbonate (PC), fluoroethylene carbonate (FEC), and 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether (TTE). This low-concentration electrolyte not only reduces cost but also delivers rapid ion diffusion and superior wetting properties. While the Na||FePO4 system with this electrolyte demonstrates slightly reduced performance at room temperature compared to standard-concentration formulations (S-PFT), it excels at both high (55 °C) and low (−20 °C) temperatures, showcasing its balanced performance. At 0.5 C (charge)/1 C (discharge), capacity retention reaches 92.8% at room temperature and 98.5% at elevated temperature, with CE values surpassing 99% and 99.63%, respectively, and significant performance sustained at −20 °C at 0.2 C. This electrolyte development thus offers a well-rounded, economically viable path to high-performance SMBs for diverse environmental applications.
Chih-Hsuan Hung et al 2025 J. Electrochem. Soc.
Long-range electric vehicles (EVs) require high-energy-density batteries that also meet the power demands of high current charge and discharge. Ultra-thick (>100 µm) Lithium-ion battery electrodes are critical to enable this need, but slow ion transport in conventional uniform electrodes (UEs) reduces battery capacity at increasing charge/discharge rates. We present a 3D computational analysis on the impact of structured electrode (SE) and graded electrode (GE) geometries on the discharge rate capability of ultra-thick graphite|LiNi0.6Mn0.2Co0.2O2 (NMC-622) battery cells based on the footprint of a commercial EV pouch cell. SE cathodes with either a “grid” or “line” geometry and GEs with two layers of porosity were modeled. Based on the results of 230 models, we found that the electrolyte volume fraction is a key parameter that impacts capacity improvements in UEs, GEs, and SEs at 2C – 6C discharge rates. SEs have the greatest discharge rate capability, outperforming GEs and UEs due to reduced Lithium-ion concentration gradients across the electrode thickness, which mitigates electrolyte depletion at high rates. The best SE model has a “grid” geometry with gravimetric and volumetric energy density improvements of 0.9% – 4% at C/2 – 2C and 18% – 24% at 4C – 6C relative to UEs.
Weiwei Xu et al 2024 J. Electrochem. Soc. 171 120545
The high cost and uneven distribution of lithium resources have prompted searches for alternatives to lithium-ion batteries. Among various alternatives, the sodium layered oxide cathode materials, have shown significant research potential due to their low cost. Layered oxide materials can be categorized into sodium-rich O3 types and sodium-deficient P2 types, which have different structural features. O3 type materials offer high specific capacities but suffer from complex pathways for Na+ de-intercalation, slow Na+ diffusion, and poor air stability. P2 type materials are limited in full cell applications due to their lower practical specific capacities. Therefore, researchers conceived the idea of combining the advantages of both to construct P2/O3 composite structure cathode materials (CSMs), utilizing the synergistic effects of the CSMs to overcome the limitations of single structure material, and successfully synthesized CSMs with appropriate specific capacities. These materials effectively suppress unfavorable phase transitions and enhance Na+ diffusion coefficient, thereby improving electrochemical performance. This paper reviews the recent advancements in CSMs for sodium-ion batteries, highlighting synthesis strategies that incorporate “cationic potential” theory, element substitution, sodium content adjustment, and control of calcination processes to synthesize diverse CSMs.
Yuhong Lan et al 2024 J. Electrochem. Soc. 171 120544
A mechano-electrochemical model is proposed to study diffusion-induced stresses (DISs) in the electrode particles with three different shapes. The governing equations of mechanical equilibrium and diffusion in spherical, cylindrical, and Cartesian coordinate systems are derived utilizing the finite deformation theory. The results of numerical simulations indicate that different shapes of particles significantly influence both the diffusion velocity and DISs. Within the same volume, the cube can save about 16% charging time compared to the sphere. The maximum value of radial stress in the cylinder is 70% smaller than that in the sphere. The hoop stress is largest in the cubic particle and smallest in the cylindrical particle.
Sarla K. Pawar et al 2024 J. Electrochem. Soc. 171 127517
This study presents a novel approach to electrochemical detection of orthophosphate in potable water using hydrothermally synthesized nickel foam electrodes, modified with cobalt oxide (CoOx), zinc oxide (ZnOx), and zinc-cobalt oxide (ZnCoOx) nanocomposites. The incorporation of zinc-cobalt oxide into the electrode significantly enhances its stability and detection capability. In-situ phosphate detection was performed using voltammetric techniques in a highly alkaline environment (pH 14) facilitated by sodium hydroxide (NaOH). Comprehensive electrode characterization, including cyclic voltammetry (0 to 0.6 V at a scan rate of 50 mV s−1), electrochemical impedance spectroscopy (105 to 10–2 Hz), scanning electron microscopy, and energy-dispersive X-ray spectroscopy, confirmed the superior performance of the synthesized electrodes. Among the tested configurations, the zinc-cobalt oxide nanocomposite on nickel foam exhibited outstanding sensitivity, with a sensitivity of 0.4 μA/μM across a concentration range of 0 to 40 μM, and an elevated sensitivity of 4.26 μA/μM within the 50 to 100 μM range. The electrode achieved a remarkably low detection limit of 0.171 μM L−1 (0 to 40 μM detection range) and 0.324 μM L−1 (50 to 100 μM, detection range), underscoring its potential for highly accurate phosphate detection. These findings highlight the sensor’s applicability for diverse practical uses in water quality monitoring.
Amir-Sina Hamedi et al 2024 J. Electrochem. Soc. 171 120539
Silicon has a remarkably high specific capacity as a Li-ion battery anode material; however, its large volume expansion and contraction make it extremely challenging to use. This work introduces a pseudo-2D (P2D or Newman-type) model that incorporates the distinctive mechanical and electrochemical behaviors of porous electrodes with large volume changes characteristic of silicon and similar active materials. Localized volume change is propagated rigorously to other electrode variables, considering elastic, plastic, and chemical strains; associated advection and hysteresis; the presence of a fluid reservoir and packaging adjacent to the cell stack; nonlinear electrode swelling behavior; deactivation of active material; and the effect of stress on open circuit potential. A silicon half-cell model is carefully parameterized by previously published experiments, and indeed provides insights in how to interpret the experiments and shows where some are problematic. The model is used as a digital twin to predict the degree of electrode utilization for different packaging designs and active material loadings, thereby allowing improved cell design.
Marko Firm et al 2024 J. Electrochem. Soc. 171 120543
Here, we extend the general approach shown in PART-1 to a detailed analysis of the measured data using our previously published physics-based transmission line model (TLM). We construct general relationships between the TLM parameters and the electrode mass. We then systematically check the scaling properties of all model parameters by fitting the measured impedance responses of 8 NMC-NMC cells with different masses (thicknesses) of NMC cathodes ranging from 2.3 to 58.5 mg per 2 cm2 of geometric electrode surface. We consider in detail all deviations of the measured spectra from the idealized spectra predicted by the model. Among other things, we discuss the high-frequency inductive effects, the ambiguities in the analysis of the low-frequency diffusion phenomena, and the effects of non-ideal capacitive properties (the so-called constant-phase elements) on the shape of the measured spectra. We also report an unexpected additional feature observed in measurements on thin (light) electrodes: a diffusion arc due to the diffusion of lithium in the electrolyte in the micropores of the NMC aggregates in the material used. Finally, we present a detailed dependence of all model parameters on the mass (thickness) of the electrode and discuss the potential practical significance of such an analysis.